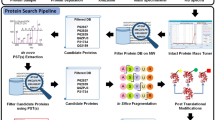
Abstract
Recently, new software tools have been developed for improved protein quantification using mass spectrometry (MS) data. However, there are still limitations especially in high-sample-throughput quantification methods, and most of these relate to extensive computational calculations. The mass accuracy precursor alignment (MAPA) strategy has been shown to be a robust method for relative protein quantification. Its major advantages are high resolution, sensitivity and sample throughput. Its accuracy is data dependent and thus best suited for precursor mass-to-charge precision of ∼1 p.p.m. This protocol describes how to use a software tool (ProtMAX) that allows for the automated alignment of precursors from up to several hundred MS runs within minutes without computational restrictions. It comprises features for 'ion intensity count' and 'target search' of a distinct set of peptides. This procedure also includes the recommended MS settings for complex quantitative MAPA analysis using ProtMAX (http://www.univie.ac.at/mosys/software.html).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Michalski, A., Cox, J. & Mann, M. More than 100,000 detectable peptide species elute in single shotgun proteomics runs but the majority is inaccessible to data-dependent LC-MS/MS. J. Proteome Res. 10, 1785–1793 (2011).
Michalski, A. et al. Mass spectrometry-based proteomics using Q Exactive, a high-performance benchtop quadrupole Orbitrap mass spectrometer. Mol. Cell. Proteomics 10, M111.011015 (2011).
Thakur, S.S. et al. Deep and highly sensitive proteome coverage by LC-MS/MS without prefractionation. Mol. Cell Proteomics 10, M110.003699 (2011).
Griffin, N.M. et al. Label-free, normalized quantification of complex mass spectrometry data for proteomic analysis. Nat. Biotechnol. 28, 83–89 (2010).
Ishihama, Y. et al. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell Proteomics 4, 1265–1272 (2005).
Liu, H., Sadygov, R.G. & Yates, J.R. A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal. Chem. 76, 4193–4201 (2004).
Lu, P., Vogel, C., Wang, R., Yao, X. & Marcotte, E.M. Absolute protein expression profiling estimates the relative contributions of transcriptional and translational regulation. Nat. Biotechnol. 25, 117–124 (2007).
Paoletti, A.C. et al. Quantitative proteomic analysis of distinct mammalian Mediator complexes using normalized spectral abundance factors. Proc. Natl. Acad. Sci. USA 103, 18928–18933 (2006).
Schulze, W.X. & Usadel, B. Quantitation in mass-spectrometry-based proteomics. Annu. Rev. Plant Biol. 61, 491–516 (2010).
Schwanhausser, B. et al. Global quantification of mammalian gene expression control. Nature 473, 337–342 (2011).
Silva, J.C., Gorenstein, M.V., Li, G.Z., Vissers, J.P.C. & Geromanos, S.J. Absolute quantification of proteins by LCMSE—a virtue of parallel MS acquisition. Mol. Cell Proteomics 5, 144–156 (2006).
Baerenfaller, K. et al. Genome-scale proteomics reveals Arabidopsis thaliana gene models and proteome dynamics. Science 320, 938–941 (2008).
Brunner, E. et al. A high-quality catalog of the Drosophila melanogaster proteome. Nat. Biotechnol. 25, 576–583 (2007).
de Godoy, L.M.F. et al. Comprehensive mass-spectrometry-based proteome quantification of haploid versus diploid yeast. Nature 455, 1251–1260 (2008).
Graumann, J. et al. Stable isotope labeling by amino acids in cell culture (SILAC) and proteome quantitation of mouse embryonic stem cells to a depth of 5,111 proteins. Mol. Cell Proteomics 7, 672–683 (2008).
Malmstrom, J. et al. Proteome-wide cellular protein concentrations of the human pathogen Leptospira interrogans. Nature 460, 762–U112 (2009).
Nagaraj, N. et al. Deep proteome and transcriptome mapping of a human cancer cell line. Mol. Syst. Biol. 7, 548 (2011).
Pavelka, N. et al. Statistical similarities between transcriptomics and quantitative shotgun proteomics data. Mol. Cell Proteomics 7, 631–644 (2008).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
De Vos, R.C.H. et al. Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2, 778–791 (2007).
Hoehenwarter, W. et al. A rapid approach for phenotype-screening and database independent detection of cSNP/protein polymorphism using mass accuracy precursor alignment. Proteomics 8, 4214–4225 (2008).
Hoehenwarter, W. et al. MAPA distinguishes genotype-specific variability of highly similar regulatory protein isoforms in potato tuber. J. Proteome Res. 10, 2979–2991 (2011).
Chen, Y., Hoehenwarther, W. & Weckwerth, W. Comparative analysis of phytohormone-responsive phosphoproteins in Arabidopsis thaliana using TiO2-phosphopeptide enrichment and mass accuracy precursor alignment. Plant J. 63, 1–17 (2010).
Doerfler, H. et al. Granger causality in integrated GC-MS and LC-MS metabolomics data reveals the interface of primary and secondary metabolism. Metabolomics. http://dx.doi.org/10.1007/s11306-012-0470-0 (25 October 2012).
Mari, A. et al. Phytochemical composition of Potentilla anserina L. analyzed by an integrative GC-MS and LC-MS metabolomics platform. Metabolomics. http://dx.doi.org/10.1007/s11306-012-0473-x (17 November 2012).
Lee, K.A., Farnsworth, C., Yu, W. & Bonilla, L.E. 24-hour lock mass protection. J. Proteome Res. 10, 880–885 (2011).
Isaacson, T. et al. Sample extraction techniques for enhanced proteomic analysis of plant tissues. Nat. Protoc. 2, 769–774 (2006).
Sheoran, I.S. et al. Compatibility of plant protein extraction methods with mass spectrometry for proteome analysis. Plant Sci. 176, 99–104 (2009).
Sun, X. & Weckwerth, W. COVAIN: a toolbox for uni- and multivariate statistics, time-series and correlation network analysis and inverse estimation of the differential Jacobian from metabolomics covariance data. Metabolomics 8, 81–93 (2012).
Acknowledgements
We thank J. Hummel for interesting comments.
Author information
Authors and Affiliations
Contributions
V.E. developed the algorithm of the ProtMAX tool. W.H. contributed to the concept and optimization of the ProtMAX tool of protein analysis and writing of the manuscript. D.L. contributed to the concept of the ProtMAX tool and writing of the manuscript. W.W. conceived the concept of the MAPA strategy for proteomics. S.W. conceived Preferences and feature upgrading with the target approach of the ProtMAX tool and was responsible for project coordination and writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Egelhofer, V., Hoehenwarter, W., Lyon, D. et al. Using ProtMAX to create high-mass-accuracy precursor alignments from label-free quantitative mass spectrometry data generated in shotgun proteomics experiments. Nat Protoc 8, 595–601 (2013). https://doi.org/10.1038/nprot.2013.013
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2013.013
This article is cited by
-
Quantitative phosphoproteomics reveals the role of the AMPK plant ortholog SnRK1 as a metabolic master regulator under energy deprivation
Scientific Reports (2016)
-
Pollen proteomics: from stress physiology to developmental priming
Plant Reproduction (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.