Abstract
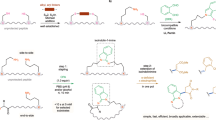
This protocol describes a convergent synthesis of reduced amide bond peptidomimetics using thioacid-terminated peptides and aziridine-containing peptide conjugates. This approach could be used to produce peptides and proteins with modified backbones. The peptide conjugates are made using readily available aziridine aldehydes. The convergent synthesis of peptidomimetics is demonstrated through the preparation of long and short peptide fragments with an aminomethylene group incorporated within them. This transformation is amenable to the synthesis of peptides with reduced amide bonds at cysteine and alanine. The procedure describes the preparation of each component used and highlights the ease of synthesis of aminomethylene peptidomimetics, and takes about 3 d to complete.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rink, R. et al. To protect peptide pharmaceuticals against peptidases. J. Pharmacol. Toxicol. Methods 61, 210–218 (2010).
Bursavich, M.G. & Rich, D.H. Designing non-peptide peptidomimetics in the 21st century: inhibitors targeting conformational ensembles. J. Med. Chem. 45, 541–558 (2002).
Inokuchi, E. et al. Design and synthesis of amidine-type peptide bond isosteres: application of nitrile oxide derivatives as active ester equivalents in peptide and peptidomimetics synthesis. Org. Biomol. Chem. 9, 3421–3427 (2011).
Martinez, J. et al. A structural modification which transforms an agonist molecule into an antagonist molecule in gastrin-like peptides. A hypothetical approach to the mechanism of action. Compt. Rend. Acad. Sci. 300, 437–440 (1985).
Szelke, M. et al. Potent new inhinitors of human renin. Nature 299, 555–557 (1982).
Grand, V., Aubry, A., Dupont, V., Vicherat, A. & Marraud, M. Folded structures in protonated reduced dipeptides. J. Peptide Sci. 2, 381–391 (1996).
Santagada, V. et al. A valuable synthesis of reduced peptide bond by microwave irradiation. QSAR Comb. Sci. 23, 899–901 (2004).
Oh, J.-E. & Lee, K.-H. Characterization of the unique function of a reduced amide bond in a cytolytic peptide that acts on phospholipid membranes. Biochem. J. 352, 659–666 (2000).
Kim, S.-M., Kim, J.-M., Cho, H. & Lee, K.-H. Synthesis of antibacterial pseudopeptides with less hemolytic activity from a cytotoxic peptide and their pH-dependent activity. Bioorg. Med. Chem. Lett. 19, 5627–5631 (2009).
Long, S.B., Hancock, P.J., Kral, A.M., Hellinga, H.W. & Beese, L.S. The crystal structure of human protein farnesyltransferase reveal the basis for inhibition by CaaX tetrapeptides and their mimetics. Proc. Natl. Acad. Sci. 98, 12948–12953 (2001).
Wlodawer, A. & Erickson, J.W. Structure-based inhibitors of HIV-1 protease. Annu. Rev. Biochem. 62, 543–585 (1993).
Weber, J. et al. Potency comparison of peptidomimetic inhibitors against HIV-1 and HIV-2 proteinases: design of equipotent lead compounds. Arch. Biochem. Biophys. 341, 62–69 (1997).
Carreno, L.F., Alba, M.P., Varela, Y., Patarroyo, M.E. & Lozano, J.M. A new approach to obtaining N-α-t-Boc-amino acid aldehydes from asparagine and glutamine for reduced amide pseudopeptide solid-phase synthesis. Chem. Biol. Drug Des. 78, 603–611 (2011).
Park, M.-S., Oh, H.-S., Cho, H. & Lee, K.-H. Microwave-assisted solid-phase synthesis of pseudopeptides containing reduced amide bond. Tetrahedron Lett. 48, 1053–1057 (2007).
Dugger, R.W., Ragan, J.A. & Ripin, D.H.B. Survey of GMP bulk reactions run in a research facility between 1985 and 2002. Org. Process Res. Dev. 9, 253–258 (2005).
Potetinova, J.V., Milgotina, E.I., Makarov, V.A. & Voyushina, T.L. Synthesis of modified peptides with C-terminal α-amino aldehydes. Russ. J. Bioorg. Chem. 27, 141–150 (2001).
Hili, R. & Yudin, A.K. Readily available unprotected amino aldehydes. J. Am. Chem. Soc. 128, 14772–14773 (2006).
Li, X. & Yudin, A.K. Epimerization- and protecting-group-free synthesis of peptidomimetic conjugates from amphoteric amino aldehydes. J. Am. Chem. Soc. 129, 14152–14153 (2007).
Dawson, P.E., Muir, T.W., Clack-Lewis, I. & Kent, S.B.H. Synthesis of proteins by native chemical ligation. Science 266, 776–779 (1994).
Wieland, V.T., Bokelmann, E., Bauer, L., Lang, H.U. & Lau, H. Über Peptidsynthesen. 8. Mitteilung Bildung von S-haltigen Peptiden durch intramolekulare Wanderung von Aminoacylresten. Annalen der Chemie 583, 129–149 (1953).
Wang, P. et al. N-linked glycopolypeptides. J. Am. Chem. Soc. 133, 1597–1602 (2011).
Crich, D. & Sana, K. Solid-phase synthesis of peptidyl thioacids employing a 9-fluorenylmethyl thioester-based linker in conjunction with Boc chemistry. J. Org. Chem. 74, 7383–7388 (2009).
Assem, N., Natarajan, A. & Yudin, A.K. Chemoselective peptidomimetic ligation using thioacid peptides and aziridine templates. J. Am. Chem. Soc. 132, 10986–10987 (2010).
Yan, L.Z. & Dawson, P.E. Synthesis of peptides and proteins without cysteine residues by native chemical ligation combined with desulfurization. J. Am. Chem. Soc. 123, 526–533 (2001).
Wan, Q. & Danishefsky, S.J. Free-radical-based, specific desulfurization of cysteine: a powerful advance in the synthesis of polypeptides and glycopolypeptides. Angew. Chem. Int. Ed. 46, 9248–9252 (2007).
Rotstein, B.H., Rai, V., Hili, R. & Yudin, A.K. Synthesis of peptide macrocycles using unprotected amino aldehydes. Nat. Protoc. 5, 1813–1822 (2010).
Acknowledgements
We thank the Natural Sciences and Engineering Research Council of Canada (NSERC) for financial support.
Author information
Authors and Affiliations
Contributions
N.A. and A.K.Y. performed the work and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Assem, N., Yudin, A. Convergent synthesis of aminomethylene peptidomimetics. Nat Protoc 7, 1327–1334 (2012). https://doi.org/10.1038/nprot.2012.066
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2012.066
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.