Abstract
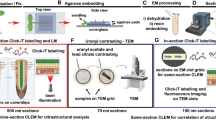
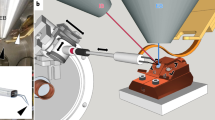
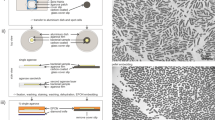
Despite improvements in live imaging, fixation followed by embedding and sectioning for light or electron microscopy remains an indispensible approach in biology. During processing, small or delicate samples can be lost, damaged or poorly oriented. Here we present a protocol for overcoming these issues when, along with chemical fixation, the sample is fixed mechanically. The protocol features two alternatives for mechanical fixation: the sample is encased either in a rectangular block of agarose or between Formvar films suspended on a wire loop. We also provide methods for key steps all the way through to sectioning. We illustrate the method on the root of Arabidopsis thaliana, an object that is ∼0.15 mm in diameter and difficult to process conventionally. With this protocol, well-oriented sections can be obtained with excellent ultrastructural preservation. The protocol takes about 1 week.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Dolan, L. et al. Cellular organisation of the Arabidopsis thaliana root. Development 119, 71–84 (1993).
Petersson, S.V. et al. An auxin gradient and maximum in the Arabidopsis root apex shown by high-resolution cell-specific analysis of IAA distribution and synthesis. Plant Cell 21, 1659–1668 (2009).
Brady, S.M. et al. A high-resolution root spatiotemporal map reveals dominant expression patterns. Science 318, 801–806 (2007).
Nawy, T. et al. Transcriptional profile of the Arabidopsis root quiescent center. Plant Cell 17, 1908–1925 (2005).
De Smet, I. et al. Bimodular auxin response controls organogenesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 107, 2705–2710 (2010).
Oorschot, V., de Wit, H., Annaert, W.G. & Klumperman, J. A novel flat-embedding method to prepare ultrathin cryosections from cultured cells in their in situ orientation. J. Histochem. Cytochem. 50, 1067–1080 (2012).
Beeckman, T. & Viane, R. Embedding thin plant specimens for oriented sectioning. Biotech. Histochem. 75, 23–26 (2000).
De Smet, I. et al. An easy and versatile embedding method for transverse sections. J. Microsc. 213, 76–80 (2004).
Leroux, O., Van der Kinderen, G. & Viane, R.L. A sandwich-embedding method for oriented sectioning. J. Microsc. 227, 79–82 (2007).
Di Laurenzio, L. et al. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell 86, 423–433 (1996).
Glauert, A.M. & Lewis, P.R. Biological Specimen Preparation for Transmission Electron Microscopy (Portland Press, London, UK, 1998).
Ruzin, S.E. Plant Microtechnique and Microscopy (Oxford University Press, New York, NY, 1999).
Baskin, T.I. & Wilson, J.E. Inhibitors of protein kinases and phosphatases alter root morphology and disorganize cortical microtubules. Plant Physiol. 113, 493–502 (1997).
Andème-Onzighi, C., Sivaguru, M., Judy-March, J., Baskin, T.I. & Driouich, A. The reb1-1 mutation of Arabidopsis alters the morphology of trichoblasts, the expression of arabinogalactan proteins and the organization of cortical microtubules. Planta 215, 949–958 (2002).
Wu, S. et al. A conditional mutation in Arabidopsis thaliana separase induces chromosome non-disjunction, aberrant morphogenesis and cyclin B1;1 stability. Development 137, 953–961 (2010).
Koizumi, K., Wu, S., MacRae-Crerar, A. & Gallagher, K.L. An essential protein that interacts with endosomes and promotes movement of the SHORT-ROOT transcription factor. Curr. Biol. 21, 1559–1564 (2011).
Vatén, A. et al. Callose biosynthesis regulates symplastic trafficking during root development. Dev. Cell 21, 1144–1155 (2011).
Graham, L. & Orenstein, J.M. Processing tissues and cells for transmission electron microscopy in diagnostic pathology and research. Nat. Protoc. 2, 2439–2450 (2007).
Lancelle, S.A., Callaham, D.A. & Hepler, P.K. A method for rapid freeze fixation of plant cells. Protoplasma 131, 153–165 (1986).
Baskin, T.I., Miller, D.D., Vos, J.W., Wilson, J.E. & Hepler, P.K. Cryofixing single cells and multicellular specimens enhances structure and immunocytochemistry for light microscopy. J. Microsc. 182, 149–161 (1996).
Newman, G.R. & Hobot, J.A. Resins for combined light and electron microscopy: a half century of development. Histochem. J. 31, 495–505 (1999).
Ellis, E.A. Solutions to the problem of substitution of ERL 4221 for vinyl cyclohexene dioxide in Spurr low viscosity embedding formulations. Microsc. Today 14, 32–33 (2006).
Groos, S., Reale, E. & Luciano, L. Re-evaluation of epoxy resin sections for light and electron microscopic immunostaining. J. Histochem. Cytochem. 49, 397–406 (2001).
Kiernan, J.A. Formaldehyde, formalin, paraformaldehyde and glutaraldehyde: What they are and what they do. Microsc. Today 8, 8–12 (2000).
Acknowledgements
We thank T. Svitkina for electron microscopy expertise, and E.P. Eleftheriou and R.S. Poethig for technical assistance and comments. S.W. is partially supported by National Science Foundation grant 0920327 awarded to K.L.G. The Formvar loop method was developed with support from the Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the United States Department of Energy through grant DE-FG-03ER15421 (to T.I.B).
Author information
Authors and Affiliations
Contributions
S.W., T.I.B. and K.L.G. developed the protocol and designed the experiments. S.W. performed the experiments. S.W., T.I.B. and K.L.G. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Video 1
Mechanical fixation: agarose method. (MOV 7548 kb)
Supplementary Video 2
Mechanical fixation: Formvar wire loop method. Green coated copper wire was used in the movie to decrease reflection and increase contrast for viewing. We recommend the use of bare copper wire in the actual experiments. (MOV 9336 kb)
Rights and permissions
About this article
Cite this article
Wu, S., Baskin, T. & Gallagher, K. Mechanical fixation techniques for processing and orienting delicate samples, such as the root of Arabidopsis thaliana, for light or electron microscopy. Nat Protoc 7, 1113–1124 (2012). https://doi.org/10.1038/nprot.2012.056
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2012.056
This article is cited by
-
Plant mitochondrial FMT and its mammalian homolog CLUH controls development and behavior in Arabidopsis and locomotion in mice
Cellular and Molecular Life Sciences (2022)
-
A non-classical route of efficient plant uptake verified with fluorescent nanoparticles and root adhesion forces investigated using AFM
Scientific Reports (2020)
-
Preparation of the planarian Schmidtea mediterranea for high-resolution histology and transmission electron microscopy
Nature Protocols (2014)
-
The differences in physiological responses, ultrastructure changes, and Na+ subcellular distribution under salt stress among the barley genotypes differing in salt tolerance
Acta Physiologiae Plantarum (2014)
-
The influence of salinity on cell ultrastructures and photosynthetic apparatus of barley genotypes differing in salt stress tolerance
Acta Physiologiae Plantarum (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.