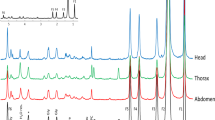
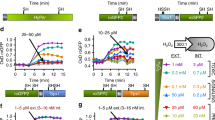
Abstract
The role of hydrogen peroxide (H2O2) in mitochondrial oxidative damage and redox signaling is poorly understood, because it is difficult to measure H2O2 in vivo. Here we describe a method for assessing changes in H2O2 within the mitochondrial matrix of living Drosophila. We use a ratiometric mass spectrometry probe, MitoB ((3-hydroxybenzyl)triphenylphosphonium bromide), which contains a triphenylphosphonium cation component that drives its accumulation within mitochondria. The arylboronic moiety of MitoB reacts with H2O2 to form a phenol product, MitoP. On injection into the fly, MitoB is rapidly taken up by mitochondria and the extent of its conversion to MitoP enables the quantification of H2O2. To assess MitoB conversion to MitoP, the compounds are extracted and the MitoP/MitoB ratio is quantified by liquid chromatography–tandem mass spectrometry relative to deuterated internal standards. This method facilitates the investigation of mitochondrial H2O2 in fly models of pathology and metabolic alteration, and it can also be extended to assess mitochondrial H2O2 production in mouse and cell culture studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 417, 1–13 (2009).
Finkel, T. & Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 408, 239–247 (2000).
Balaban, R.S., Nemoto, S. & Finkel, T. Mitochondria, oxidants, and aging. Cell 120, 483–495 (2005).
Rhee, S.G. et al. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr. Opin. Cell Biol. 17, 183–189 (2005).
Murphy, M.P. et al. Unraveling the biological roles of reactive oxygen species. Cell Metab 13, 361–366 (2011).
Dickinson, B.C. & Chang, C.J. A targetable fluorescent probe for imaging hydrogen peroxide in the mitochondria of living cells. J. Am. Chem. Soc. 130, 9638–9639 (2008).
Miller, E.W., Tulyathan, O., Isacoff, E.Y. & Chang, C.J. Molecular imaging of hydrogen peroxide produced for cell signaling. Nat. Chem. Biol. 3, 263–267 (2007).
Belousov, V.V. et al. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat. Methods 3, 281–286 (2006).
Beckman, K.B. & Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 78, 547–581 (1998).
Chance, B., Sies, H. & Boveris, A. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 59, 527–605 (1979).
Oshino, N., Jamieson, D. & Chance, B. The properties of hydrogen peroxide production under hyperoxic and hypoxic conditions of perfused rat liver. Biochem. J. 146, 53–65 (1975).
Cochemé, H.M. et al. Measurement of H2O2 within living Drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab. 13, 340–350 (2011).
Murphy, M.P. & Smith, R.A.J. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 47, 629–656 (2007).
Ross, M.F. et al. Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochemistry (Mosc) 70, 222–230 (2005).
Kuivila, H.G. & Armour, A.G. Electrophilic displacement reactions. IX. Effects of substituents on rates of reactions between hydrogen peroxide and benzeneboronic acid. J. Am. Chem. Soc. 79, 5659–5662 (1957).
Sikora, A., Zielonka, J., Lopez, M., Joseph, J. & Kalyanaraman, B. Direct oxidation of boronates by peroxynitrite: mechanism and implications in fluorescence imaging of peroxynitrite. Free Radic. Biol. Med. 47, 1401–1407 (2009).
Szabo, C., Ischiropoulos, H. & Radi, R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat. Rev. 6, 662–680 (2007).
Barry, S.J. et al. Use of S-pentafluorophenyl tris(2,4,6-trimethoxyphenyl)phosphonium acetate bromide and (4-hydrazino-4-oxobutyl) [tris(2,4,6-trimethoxyphenyl)phosphonium bromide for the derivatization of alcohols, aldehydes and ketones for detection by liquid chromatography/electrospray mass spectrometry. Rapid Commun. Mass Spectrom. 17, 484–497 (2003).
Woo, H.K. et al. Phosphonium labeling for increasing metabolomic coverage of neutral lipids using electrospray ionization mass spectrometry. Rapid. Commun. Mass Spectrom. 23, 1849–1855 (2009).
Yue Qian, S., Kadiiska, M.B., Guo, Q. & Mason, R.P. A novel protocol to identify and quantify all spin trapped free radicals from in vitro/in vivo interaction of HO. and DMSO: LC/ESR, LC/MS, and dual spin trapping combinations. Free Radic. Biol. Med. 38, 125–135 (2005).
Reis, A., Domingues, M.R.M., Oliveira, M.M. & Domingues, P. Identification of free radicals by spin trapping with DEPMPO and MCPIO using tandem mass spectrometry. Eur. J. Mass Spec. 15, 689–703 (2009).
Meyer, A.J. & Dick, T.P. Fluorescent protein-based redox probes. Antioxid. Redox. Signal 13, 621–50 (2010).
Kuzin, B., Roberts, I., Peunova, N. & Enikolopov, G. Nitric oxide regulates cell proliferation during Drosophila development. Cell 87, 639–649 (1996).
Regulski, M. & Tully, T. Molecular and biochemical characterization of dNOS: a Drosophila Ca2+/calmodulin-dependent nitric oxide synthase. Proc. Natl. Acad. Sci. USA 92, 9072–9076 (1995).
Claereboudt, J., Baeten, W., Geise, H. & Claeys, M. Structural characterization of mono- and bisphosphonium salts by fast atom bombardment mass spectrometry and tandem mass spectrometry. Org. Mass Spec. 28, 71–82 (1993).
Denekamp, C., Pocsfalvi, G. & Claeys, M. Charge-remote and charge-proximate fragmentations in deuterium-labeled n-hexyadecyltriphenylphosphonium cations. Int. J. Mass Spec. 188, 163–175 (1999).
Denekamp, C., Tenetov, E. & Horev, Y. Homolytic cleavages in pyridinium ions, an excited state process. J. Am. Soc. Mass Spec. 14, 790–801 (2003).
Magwere, T. et al. The effect of dietary restriction on mitochondrial protein density and flight muscle mitochondrial morphology in Drosophila. J. Gerontol. A Biol. Sci. Med. Sci. 61, 36–47 (2006).
Porteous, C.M. et al. Rapid uptake of lipophilic triphenylphosphonium cations by mitochondria in vivo following intravenous injection: implications for mitochondria-specific therapies and probes. Biochimica. Biophysica. Acta 1800, 1009–1017 (2010).
Acknowledgements
This work was supported by the UK Biotechnology and Biological Sciences Research Council, the UK Medical Research Council, the Wellcome Trust, the New Zealand Foundation for Research Science and Technology, the University of Glasgow (to C.Q.), the United Mitochondrial Disease Foundation, the Lloyds TSB Foundation for Scotland and the Royal Society of Edinburgh (to S.J.M.).
Author information
Authors and Affiliations
Contributions
H.M.C., M.P.M., R.A.J.S., L.P. and R.C.H. designed the research; H.M.C., T.A.P., A.L., I.A., C.Q., S.J.M., J.V.P. and C.M.P. carried out the experiments; H.M.C., M.P.M., A.M.J. and I.M.F. analyzed data; H.M.C., A.L. and M.P.M. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Video 1
Video of a fly being injected with MitoB. A female fly is aneasthetised on a CO2 pad, then a glass microinjector needle containing the MitoB solution supplemented with blue dye is inserted into the thorax, using a fine-haired paintbrush to position and support the fly. The injection pulse is triggered by a foot pedal, and the blue dye rapidly diffuses throughout the fly body. (MOV 7744 kb)
Rights and permissions
About this article
Cite this article
Cochemé, H., Logan, A., Prime, T. et al. Using the mitochondria-targeted ratiometric mass spectrometry probe MitoB to measure H2O2 in living Drosophila. Nat Protoc 7, 946–958 (2012). https://doi.org/10.1038/nprot.2012.035
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2012.035
This article is cited by
-
Engineering a polymer-encapsulated manganese dioxide/upconversion nanoprobe for FRET-based hydrogen peroxide detection
Analytical and Bioanalytical Chemistry (2023)
-
Hepatic resistance to cold ferroptosis in a mammalian hibernator Syrian hamster depends on effective storage of diet-derived α-tocopherol
Communications Biology (2021)
-
Egr-1 functions as a master switch regulator of remote ischemic preconditioning-induced cardioprotection
Basic Research in Cardiology (2020)
-
Mitochondria as a therapeutic target for common pathologies
Nature Reviews Drug Discovery (2018)
-
Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection
Basic Research in Cardiology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.