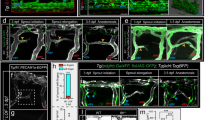
Abstract
Formation of new blood and lymphatic vessels is involved in many physiological and pathological processes, including organ and tumor growth, cancer cell metastasis, fluid drainage and lymphedema. Therefore, the ability to manipulate vascularization in a mammalian system is of particular interest to researchers. Here we describe a method for pharmacological manipulation of de novo and sprouting blood and lymphatic vascular development in ex vivo–cultured mouse embryos. The described protocol can also be used to evaluate the properties of pharmacological agents in growing mammalian tissues and to manipulate other developmental processes. The whole procedure, from embryo isolation to image quantification, takes 3–5 d, depending on the analysis and age of the embryos.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ferrara, N. et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380, 439–442 (1996).
Carmeliet, P. et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380, 435–439 (1996).
Duarte, A. et al. Dosage-sensitive requirement for mouse Dll4 in artery development. Genes Dev. 18, 2474–2478 (2004).
Leveen, P. et al. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 8, 1875–1887 (1994).
Lindahl, P., Johansson, B.R., Leveen, P. & Betsholtz, C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277, 242–245 (1997).
Strilic, B. et al. The molecular basis of vascular lumen formation in the developing mouse aorta. Dev. Cell 17, 505–515 (2009).
Strilic, B. et al. Electrostatic cell-surface repulsion initiates lumen formation in developing blood vessels. Curr. Biol. 20, 2003–2009 (2010).
Randhawa, P.K. et al. The Ras activator RasGRP3 mediates diabetes-induced embryonic defects and affects endothelial cell migration. Circ. Res. 108, 1199–1208 (2011).
Nagase, M., Nagase, T., Koshima, I. & Fujita, T. Critical time window of hedgehog-dependent angiogenesis in murine yolk sac. Microvasc. Res. 71, 85–90 (2006).
Downs, K.M., Hellman, E.R., McHugh, J., Barrickman, K. & Inman, K.E. Investigation into a role for the primitive streak in development of the murine allantois. Development 131, 37–55 (2004).
Kawasaki, K. et al. Ras signaling directs endothelial specification of VEGFR2+ vascular progenitor cells. J. Cell Biol. 181, 131–141 (2008).
Bohnsack, B.L., Lai, L., Dolle, P. & Hirschi, K.K. Signaling hierarchy downstream of retinoic acid that independently regulates vascular remodeling and endothelial cell proliferation. Genes Dev. 18, 1345–1358 (2004).
Planas-Paz, L. et al. Mechanoinduction of lymph vessel expansion. EMBO J. 31, 788–804 (2011).
Xu, K. et al. Blood vessel tubulogenesis requires Rasip1 regulation of GTPase signaling. Dev. Cell 20, 526–539 (2011).
Parker, L.H. et al. The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature 428, 754–758 (2004).
Gerhardt, H. et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161, 1163–1177 (2003).
Wigle, J.T. & Oliver, G. Prox1 function is required for the development of the murine lymphatic system. Cell 98, 769–778 (1999).
Karkkainen, M.J. et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 5, 74–80 (2004).
Kiefer, F. & Adams, R.H. Lymphatic endothelial differentiation: start out with Sox—carry on with Prox. Genome Biol. 9, 243 (2008).
Makinen, T., Norrmen, C. & Petrova, T.V. Molecular mechanisms of lymphatic vascular development. Cell Mol. Life Sci. 64, 1915–1929 (2007).
Böhmer, R. et al. Regulation of developmental lymphangiogenesis by Syk+ leukocytes. Dev. Cell 18, 437–449 (2010).
Tammela, T. & Alitalo, K. Lymphangiogenesis: molecular mechanisms and future promise. Cell 140, 460–476 (2010).
Kälin, R.E., Banziger-Tobler, N.E., Detmar, M. & Brändli, A.W. An in vivo chemical library screen in Xenopus tadpoles reveals novel pathways involved in angiogenesis and lymphangiogenesis. Blood 114, 1110–1122 (2009).
Hellstrom, M., Kalen, M., Lindahl, P., Abramsson, A. & Betsholtz, C. Role of PDGF-B and PDGFR-β in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126, 3047–3055 (1999).
Norrmen, C. et al. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J. Cell Biol. 185, 439–457 (2009).
Sabine, A. et al. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev. Cell 22, 430–445 (2012).
Sasaki, T. et al. Regulation of hematopoietic cell clusters in the placental niche through SCF/Kit signaling in embryonic mouse. Development 137, 3941–3952 (2010).
Hang, C.T. et al. Chromatin regulation by Brg1 underlies heart muscle development and disease. Nature 466, 62–67 (2010).
Chang, C.P. et al. A field of myocardial-endocardial NFAT signaling underlies heart valve morphogenesis. Cell 118, 649–663 (2004).
Miki, R. et al. Fate maps of ventral and dorsal pancreatic progenitor cells in early somite stage mouse embryos. Mech. Dev. 128, 597–609 (2012).
Tremblay, K.D. & Zaret, K.S. Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev. Biol. 280, 87–99 (2005).
Noda, T. et al. Restriction of Wnt signaling in the dorsal otocyst determines semicircular canal formation in the mouse embryo. Dev. Biol. 362, 83–93 (2012).
Calegari, F. & Huttner, W.B. An inhibition of cyclin-dependent kinases that lengthens, but does not arrest, neuroepithelial cell cycle induces premature neurogenesis. J. Cell Sci. 116, 4947–4955 (2003).
Inoue, T. & Krumlauf, R. An impulse to the brain—using in vivo electroporation. Nat. Neurosci. 4 (suppl.): 1156–1158 (2001).
Gray, J. & Ross, M.E. Neural tube closure in mouse whole embryo culture. J. Vis. Exp. published online, doi:10.3791/3132 (2011).
Takahashi, M., Nomura, T. & Osumi, N. Transferring genes into cultured mammalian embryos by electroporation. Dev. Growth Differ. 50, 485–497 (2008).
Winn, L.M. & Tung, E.W. Assessment of embryotoxicity using mouse embryo culture. Methods Mol. Biol. 550, 241–249 (2009).
Jones, E.A. et al. Dynamic in vivo imaging of postimplantation mammalian embryos using whole embryo culture. Genesis 34, 228–235 (2002).
Kwon, G.S. et al. Tg(Afp-GFP) expression marks primitive and definitive endoderm lineages during mouse development. Dev. Dyn. 235, 2549–2558 (2006).
Schmidt, M. et al. EGFL7 regulates the collective migration of endothelial cells by restricting their spatial distribution. Development 134, 2913–2923 (2007).
Boisset, J.C., Andrieu-Soler, C., van Cappellen, W.A., Clapes, T. & Robin, C. Ex vivo time-lapse confocal imaging of the mouse embryo aorta. Nat. Protoc. 6, 1792–1805 (2011).
Drake, C.J. & Fleming, P.A. Vasculogenesis in the day 6.5 to 9.5 mouse embryo. Blood 95, 1671–1679 (2000).
Veikkola, T. et al. Signalling via vascular endothelial growth factor receptor-3 is sufficient for lymphangiogenesis in transgenic mice. EMBO J. 20, 1223–1231 (2001).
Skobe, M. et al. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat. Med. 7, 192–198 (2001).
Garcia, M.D., Udan, R.S., Hadjantonakis, A.K. & Dickinson, M.E. Preparation of rat serum for culturing mouse embryos. Cold Spring Harb. Protoc. 2011, 391–393 (2011).
Schulte-Merker, S., Sabine, A. & Petrova, T.V. Lymphatic vascular morphogenesis in development, physiology, and disease. J. Cell Biol. 193, 607–618 (2011).
Carmeliet, P. et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat. Med. 7, 575–583 (2001).
Akerman, S. et al. Microflow of fluorescently labelled red blood cells in tumours expressing single isoforms of VEGF and their response to vascular targeting agents. Med. Eng. Phys. 33, 805–809 (2011).
Fong, T.A. et al. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 59, 99–106 (1999).
Karpanen, T. et al. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 61, 1786–1790 (2001).
Bakkers, J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc. Res. 91, 279–288 (2011).
Jin, S.W., Beis, D., Mitchell, T., Chen, J.N. & Stainier, D.Y. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development 132, 5199–5209 (2005).
Patan, S. Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J. Neurooncol. 50, 1–15 (2000).
Thisse, C. & Zon, L.I. Organogenesis—heart and blood formation from the zebrafish point of view. Science 295, 457–462 (2002).
Mendel, D.B. et al. The angiogenesis inhibitor SU5416 has long-lasting effects on vascular endothelial growth factor receptor phosphorylation and function. Clin. Cancer Res. 6, 4848–4858 (2000).
Finnerty, H. et al. Molecular cloning of murine FLT and FLT4. Oncogene 8, 2293–2298 (1993).
Abramoff, M.D., Magalhaes, P.J. & Ram, S.J. Image processing with ImageJ. Biophotonics International 11, 36–42 (2004).
Walls, J.R., Coultas, L., Rossant, J. & Henkelman, R.M. Three-dimensional analysis of vascular development in the mouse embryo. PLoS ONE 3, e2853 (2008).
Acknowledgements
We are grateful to M. Gearing for proofreading the manuscript and to the other members of the Lammert laboratory for valuable discussions. This work was funded by the Deutsche Forschungsgemeinschaft (DFG) LA 1216/4-1 and 5-1, and by the Collaborative Research Centre (CRC) 974.
Author information
Authors and Affiliations
Contributions
M.Z., J.A. and E.L. wrote the text. M.Z. and J.A. prepared the figures. M.Z., J.A., B.S. and T.H. performed the blood vessel experiments. L.P.-P. and J.A. performed the lymphatic vessel experiments.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
Images to illustrate important steps of skin isolation as described in step 20C. (PDF 212 kb)
Supplementary Figure 2
Transversal sections through mouse embryos after microinjection and whole embryo culture (WEC) illustrating the local effects of injected substances. (PDF 1063 kb)
Supplementary Table 1
Survival rates of long-term WEC in M16. (PDF 38 kb)
Supplementary Table 2
Range of heart rates after WEC. (PDF 57 kb)
Supplementary Video 1
Uterus separation, isolation and injection of E8.0 mouse embryos are shown together with common mistakes. (MOV 31388 kb)
Supplementary Video 2
Uterus separation, isolation and injection of E8.75 mouse embryos are shown together with common mistakes. (MOV 14856 kb)
Supplementary Video 3
Uterus separation, isolation and injection of E11.5 mouse embryos are shown together with common mistakes. (MOV 18778 kb)
Supplementary Video 4
Uterus separation, isolation and injection of E15.5 mouse embryos are shown together with common mistakes. (MOV 14729 kb)
Supplementary Video 5
Heartbeat movie of an E8.5 mouse embryo after 12 h of WEC. (MOV 6926 kb)
Rights and permissions
About this article
Cite this article
Zeeb, M., Axnick, J., Planas-Paz, L. et al. Pharmacological manipulation of blood and lymphatic vascularization in ex vivo–cultured mouse embryos. Nat Protoc 7, 1970–1982 (2012). https://doi.org/10.1038/nprot.2012.120
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2012.120
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.