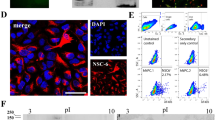
Abstract
Neural stem cells (NSCs) have the remarkable capacity to self-renew and the lifelong ability to generate neurons in the adult mammalian brain. However, the molecular and cellular mechanisms contributing to these behaviors are still not understood. Now that prospective isolation of the NSCs has become feasible, these mechanisms can be studied. Here we describe a protocol for the efficient isolation of adult NSCs, by the application of a dual-labeling strategy on the basis of their glial identity and ciliated nature. The cells are isolated from the lateral ventricular subependymal zone (SEZ) of adult hGFAP-eGFP (human glial fibrillary acidic protein–enhanced green fluorescent protein) transgenic mice by fluorescence-activated cell sorting. Staining against prominin1 (CD133) allows the isolation of the NSCs (hGFAP-eGFP+/prominin1+), which can be further subdivided by labeling with the fluorescent epidermal growth factor. This protocol, which can be completed in 7 h, allows the assessment of quantitative changes in SEZ NSCs and the examination of their molecular and functional characteristics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kriegstein, A. & Alvarez-Buylla, A. The glial nature of embryonic and adult neural stem cells. Annu. Rev. Neurosci. 32, 149–184 (2009).
Ninkovic, J. & Gotz, M. Signaling in adult neurogenesis: from stem cell niche to neuronal networks. Curr. Opin. Neurobiol. 17, 338–344 (2007).
Curtis, M.A., Kam, M. & Faull, R.L. Neurogenesis in humans. Eur. J. Neurosci. 33, 1170–1174 (2011).
Arvidsson, A., Collin, T., Kirik, D., Kokaia, Z. & Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 8, 963–970 (2002).
Brill, M.S. et al. Adult generation of glutamatergic olfactory bulb interneurons. Nat. Neurosci. 12, 1524–1533 (2009).
Carlen, M. et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat. Neurosci. 12, 259–267 (2009).
Goings, G.E., Sahni, V. & Szele, F.G. Migration patterns of subventricular zone cells in adult mice change after cerebral cortex injury. Brain Res. 996, 213–226 (2004).
Jablonska, B. et al. Chordin-induced lineage plasticity of adult SVZ neuroblasts after demyelination. Nat. Neurosci. 13, 541–550 (2010).
Beckervordersandforth, R. et al. In vivo fate mapping and expression analysis reveals molecular hallmarks of prospectively isolated adult neural stem cells. Cell Stem Cell 7, 744–758 (2010).
Pinto, L. et al. Prospective isolation of functionally distinct radial glial subtypes—lineage and transcriptome analysis. Mol. Cell Neurosci. 38, 15–42 (2008).
Weigmann, A., Corbeil, D., Hellwig, A. & Huttner, W.B. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc. Natl Acad. Sci. USA 94, 12425–12430 (1997).
Mirzadeh, Z., Merkle, F.T., Soriano-Navarro, M., Garcia-Verdugo, J.M. & Alvarez-Buylla, A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell 3, 265–278 (2008).
Nolte, C. et al. GFAP promoter-controlled EGFP-expressing transgenic mice: a tool to visualize astrocytes and astrogliosis in living brain tissue. Glia 33, 72–86 (2001).
Capela, A. & Temple, S. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron 35, 865–875 (2002).
Corti, S. et al. Isolation and characterization of murine neural stem/progenitor cells based on Prominin-1 expression. Exp. Neurol. 205, 547–562 (2007).
Kawaguchi, A. et al. Nestin-EGFP transgenic mice: visualization of the self-renewal and multipotency of CNS stem cells. Mol. Cell Neurosci. 17, 259–273 (2001).
Pastrana, E., Cheng, L.C. & Doetsch, F. Simultaneous prospective purification of adult subventricular zone neural stem cells and their progeny. Proc. Natl Acad. Sci. USA 106, 6387–6392 (2009).
Rietze, R.L. et al. Purification of a pluripotent neural stem cell from the adult mouse brain. Nature 412, 736–739 (2001).
Azari, H. et al. Purification of immature neuronal cells from neural stem cell progeny. PLoS One 6, e20941 (2011).
Barraud, P., Thompson, L., Kirik, D., Bjorklund, A. & Parmar, M. Isolation and characterization of neural precursor cells from the Sox1-GFP reporter mouse. Eur. J. Neurosci. 22, 1555–1569 (2005).
Hack, M.A., Sugimori, M., Lundberg, C., Nakafuku, M. & Gotz, M. Regionalization and fate specification in neurospheres: the role of Olig2 and Pax6. Mol. Cell Neurosci. 25, 664–678 (2004).
Gabay, L., Lowell, S., Rubin, L.L. & Anderson, D.J. Deregulation of dorsoventral patterning by FGF confers trilineage differentiation capacity on CNS stem cells in vitro. Neuron 40, 485–499 (2003).
Costa, M.R. et al. Continuous live imaging of adult neural stem cell division and lineage progression in vitro. Development 138, 1057–1068 (2011).
Ortega, F. et al. An adherent cell culture of the mouse subependymal zone to study the behavior of adult neural stem cells on a single cell level. Nat. Protoc. 6, 1847–1857 (2011).
Ciccolini, F., Mandl, C., Holzl-Wenig, G., Kehlenbach, A. & Hellwig, A. Prospective isolation of late development multipotent precursors whose migration is promoted by EGFR. Dev. Biol. 284, 112–125 (2005).
Colak, D. et al. Adult neurogenesis requires Smad4-mediated bone morphogenic protein signaling in stem cells. J. Neurosci. 28, 434–446 (2008).
Zhuo, L. et al. Live astrocytes visualized by green fluorescent protein in transgenic mice. Dev. Biol. 187, 36–42 (1997).
Malatesta, P., Hartfuss, E. & Gotz, M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development 127, 5253–5263 (2000).
Malatesta, P. et al. Neuronal or glial progeny: regional differences in radial glia fate. Neuron 37, 751–764 (2003).
Requardt, R.P. et al. Quality control of astrocyte-directed Cre transgenic mice: the benefits of a direct link between loss of gene expression and reporter activation. Glia 57, 680–692 (2009).
Shen, Q. et al. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell 3, 289–300 (2008).
Tavazoie, M. et al. A specialized vascular niche for adult neural stem cells. Cell Stem Cell 3, 279–288 (2008).
Mirzadeh, Z., Doetsch, F., Sawamoto, K., Wichterle, H. & Alvarez-Buylla, A. The subventricular zone en-face: wholemount staining and ependymal flow. J. Vis. Exp. 39 Published online, doi:10.3791/1938 (2010).
Alexander, C.M. et al. Separating stem cells by flow cytometry: reducing variability for solid tissues. Cell Stem Cell 5, 579–583 (2009).
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 870, Go640/7.1, 8.1, 9.1, SFB 596), the Bundesministerium für Bildung und Forschung, the Helmholtz association (HELMA) and the European Union (EUTRACC, EduGlia).
Author information
Authors and Affiliations
Contributions
J.F. and R.B. contributed to experimental design, execution and preparation of the manuscript. P.T. pioneered the establishment of the procedure. A.S.-M. provided support and technical assistance. J.N. provided conceptual advice, helped to design the experiments and assisted with the manuscript. M.G. developed, supervised and financed the project, and was involved in many discussions of the experimental approaches as well as in writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
(A-C) Dot plots depicting SEZ cells according to size and granularity (FCS-A vs. SSC-A; P1). (A′-C′) Dot plots illustrating cells positive for the marker of interest (for gate setting see Fig. 4). Colours depict cells expressing hGFAP-eGFP (A′; green), prominin1 (B′; red), EGFR (C′; blue) and indicate their positions according to FCS-A and SSC-A as a backprojection to P1 (A-C). Note that almost all hGFAP-GFP+, EGFR+ and most of the prominin1+ cells are located within the lower arm of the SEZ cell distribution, justifying the P1 gate in Fig 4. (PDF 147 kb)
Rights and permissions
About this article
Cite this article
Fischer, J., Beckervordersandforth, R., Tripathi, P. et al. Prospective isolation of adult neural stem cells from the mouse subependymal zone. Nat Protoc 6, 1981–1989 (2011). https://doi.org/10.1038/nprot.2011.412
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2011.412
This article is cited by
-
Dentate gyrus astrocytes exhibit layer-specific molecular, morphological and physiological features
Nature Neuroscience (2022)
-
The extracellular matrix molecule tenascin-C modulates cell cycle progression and motility of adult neural stem/progenitor cells from the subependymal zone
Cellular and Molecular Life Sciences (2022)
-
TDP-43 condensates and lipid droplets regulate the reactivity of microglia and regeneration after traumatic brain injury
Nature Neuroscience (2022)
-
Pluripotent stem cells induced from mouse neural stem cells and small intestinal epithelial cells by small molecule compounds
Cell Research (2016)
-
Age-related neurogenesis decline in the subventricular zone is associated with specific cell cycle regulation changes in activated neural stem cells
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.