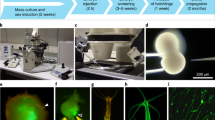
Abstract
The great regenerative abilities of newts provide the impetus for studies at the molecular level. However, efficient methods for gene regulation have historically been quite limited. Here we describe a protocol for transgenically expressing exogenous genes in the newt Cynops pyrrhogaster. This method is simple: a reaction mixture of I-SceI meganuclease and a plasmid DNA carrying a transgene cassette flanked by the enzyme recognition sites is directly injected into fertilized eggs. The protocol achieves a high efficiency of transgenesis, comparable to protocols used in other animal systems, and it provides a practical number of transgenic newts (∼20% of injected embryos) that survive beyond metamorphosis and that can be applied to regenerative studies. The entire protocol for obtaining transgenic adult newts takes 4–5 months.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Sánchez Alvarado, A. & Tsonis, P.A. Bridging the regeneration gap: genetic insights from diverse animal models. Nat. Rev. Genet. 7, 873–884 (2006).
Tsonis, P.A. Regeneration in vertebrates. Dev. Biol. 221, 273–284 (2000).
Kragl, M. et al. Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature 460, 60–65 (2009).
Del Rio-Tsonis, K. & Tsonis, P.A. Eye regeneration at the molecular age. Dev. Dyn. 226, 211–224 (2003).
Tsonis, P.A. & Del Rio-Tsonis, K. Lens and retina regeneration: transdifferentiation, stem cells and clinical applications. Exp. Eye Res. 78, 161–172 (2004).
Tsonis, P.A. et al. Controlling gene loss of function in newts with emphasis on lens regeneration. Nat. Protoc. 6, 593–599 (2011).
Casco-Robles, M.M., Yamada, S., Miura, T. & Chiba, C. Simple and efficient transgenesis with I-SceI meganuclease in the newt, Cynops pyrrhogaster. Dev. Dyn. 239, 3275–3284 (2010).
Jacquier, A. & Dujon, B. An intron-encoded protein is active in a gene conversion process that spreads an intron into a mitochondrial gene. Cell 41, 383–394 (1985).
Thermes, V. et al. I-SceI meganuclease mediates highly efficient transgenesis in fish. Mech. Dev. 118, 91–98 (2002).
Hosemann, K.E., Colosimo, P.F., Summers, B.R. & Kingsley, D.M. Simple and efficient microinjection protocol for making transgenic sticklebacks. Behaviour 141, 1345–1355 (2004).
Grabher, C., Joly, J.-S. & Wittbrodt, J. Highly efficient zebrafish transgenesis mediated by the meganuclease I-SceI. Methods Cell Biol. 77, 381–401 (2004).
Sobkow, L., Epperlein, H.-H., Hrklotz, S., Straube, W.L. & Tanaka, E.M. A germline GFP transgenic axolotl and its use to tack cell fate: dual origin of the fin mesenchyme during development and the fate of blood cells during regeneration. Dev. Biol. 290, 386–397 (2006).
Pan, F.C., Chen, Y.L., Loeber, J., Henningfeld, K. & Pieler, T. I-SceI meganuclease-mediated transgenesis in Xenopus. Dev. Dyn. 235, 247–252 (2006).
Ogino, H., McDonnell, W.B. & Grainger, R.M. High-throughput transgenesis in Xenopus using I-SceI meganuclease. Nat. Protoc. 1, 1703–1710 (2006).
Deschet, K., Nakatani, Y. & Smith, W.C. Generation of Ci-Brachyury-GFP stable transgenic lines in the ascidian Ciona Savignyi. Genesis 35, 248–259 (2003).
Ueda, Y., Kondoh, H. & Mizuno, N. Generation of transgenic newt Cynops pyrrhogaster for regeneration study. Genesis 41, 87–98 (2005).
Makita, R., Kondoh, H. & Okamoto, M. Transgenesis of newt with exogenous gene expression facilitated by satellite 2 repeats. Dev. Growth Differ. 37, 605–616 (1995).
Recillas-Targa, F. et al. Position-effect protection and enhancer blocking by the chicken β-globin insulator are separable activities. Proc. Natl Acad. Sci. USA 99, 6883–6888 (2002).
Chiba, C. et al. Visual cycle protein RPE65 persists in new retinal cells during retinal regeneration of adult newt. J. Comp. Neurol. 495, 391–407 (2006).
Acknowledgements
We are thankful to E M. Tanaka for her kind and generous gift of pCAGGs-EGFP (Sce). This work was supported by a Grant-in-Aid for Challenging Exploratory Research (20650060) and a Grant-in-Aid for Scientific Research (B) (21300150) from the Japan Society for the Promotion of Science (JSPS) to C.C. and by NIH grant EY10540 to P.A.T.
AUTHOR CONTRIBUTIONS
C.C. designed, directed and analyzed data. M.M.C.-R., S.Y., T.M., K.N., T.H., N.M. and K.D.R.-T. performed experiments and wrote part of the protocols. P.A.T. and C.C. co-wrote the final version of the paper.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Video 1. Transgenic Cynops pyrrhogaster newts generated by this protocol with pCAGGs-EGFP(Sce) construct.
The movie shows 'Uniform' EGFP-expressing swimming larvae at stage 40 and 50, and a head region, forelimbs and beating heart of another larva at stage 50, and finally a juvenile after metamorphosis. Permission by the University of Tsukuba AUCC was obtained for these experiments. This video was reproduced, with permission, from the data shown in ref. 7 ©Wiley. (WMV 20052 kb)
Rights and permissions
About this article
Cite this article
Casco-Robles, M., Yamada, S., Miura, T. et al. Expressing exogenous genes in newts by transgenesis. Nat Protoc 6, 600–608 (2011). https://doi.org/10.1038/nprot.2011.334
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2011.334
This article is cited by
-
The latent dedifferentiation capacity of newt limb muscles is unleashed by a combination of metamorphosis and body growth
Scientific Reports (2022)
-
Novel erythrocyte clumps revealed by an orphan gene Newtic1 in circulating blood and regenerating limbs of the adult newt
Scientific Reports (2018)
-
Turning the fate of reprogramming cells from retinal disorder to regeneration by Pax6 in newts
Scientific Reports (2016)
-
A developmentally regulated switch from stem cells to dedifferentiation for limb muscle regeneration in newts
Nature Communications (2016)
-
The newt (Cynops pyrrhogaster) RPE65 promoter: molecular cloning, characterization and functional analysis
Transgenic Research (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.