Abstract
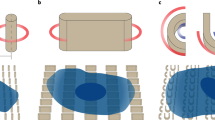
Physical inputs, both internal and external to a cell, can directly alter the spatial organization of cell surface receptors and their associated functions. Here we describe a protocol that combines solid-state nanolithography and supported lipid membrane techniques to trigger and manipulate specific receptors on the surface of living cells and to develop an understanding of the interplay between spatial organization and receptor function. While existing protein-patterning techniques are capable of presenting cells with well-defined clusters of protein, this protocol uniquely allows for the control of the spatial organization of laterally fluid receptor-ligand complex at an intermembrane junction. A combination of immunofluorescence and single-cell microscopy methods and complementary biochemical analyses are used to characterize receptor signaling pathways and cell functions. The protocol requires 2–5 d to complete depending on the parameters to be studied. In principle, this protocol is widely applicable to eukaryotic cells and herein is specifically developed to study the role of physical organization and translocation of the EphA2 receptor tyrosine kinase across a library of model breast cancer cell lines.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Singh, A.B. & Harris, R.C. Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cell Signal. 17, 1183–1193 (2005).
Salaita, K. et al. Restriction of receptor movement alters cellular response: physical force sensing by EphA2. Science 327, 1380–1385 (2010).
Mossman, K.D., Campi, G., Groves, J.T. & Dustin, M.L. Altered TCR signaling from geometrically repatterned immunological synapses. Science 310, 1191–3 (2005).
Mammen, M., Choi, S. & Whitesides, G.M. Polyvalent interactions in biological systems: implications for design and use of multivalent ligands and inhibitors. Angew. Chem. Int. Ed. Engl. 37, 2754–2794 (1998).
Discher, D.E., Janmey, P. & Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005).
del Rio, A. et al. Stretching single talin rod molecules activates vinculin binding. Science 323, 638–641 (2009).
Vogel, V. & Sheetz, M. Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 7, 265–275 (2006).
Hatzakis, N.S. et al. How curved membranes recruit amphipathic helices and protein anchoring motifs. Nat. Chem. Biol. 5, 835–841 (2009).
Demond, A.L., Mossman, K.D., Starr, T., Dustin, M.L. & Groves, J.T. T cell receptor microcluster transport through molecular mazes reveals mechanism of translocation. Biophys. J. 94, 3286–3292 (2008).
Manz, B.N. & Groves, J.T. Spatial organization and signal transduction at intercellular junctions. Nat. Rev. Mol. Cell Biol. 11, 342–352 (2010).
Hartman, N.C., Nye, J.A. & Groves, J.T. Cluster size regulates protein sorting in the immunological synapse. Proc. Natl. Acad. Sci. USA 106, 12729–12734 (2009).
Lackmann, M. & Boyd, A.W. Eph, a protein family coming of age: more confusion, insight, or complexity? Sci. Signal. 1, re2 (2008).
Kullander, K. & Klein, R. Mechanisms and functions of Eph and ephrin signalling. Nat. Rev. Mol. Cell Biol. 3, 475–86 (2002).
Davis, S. et al. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science 266, 816–9 (1994).
Muzzey, D. & van Oudenaarden, A. Quantitative time-lapse fluorescence microscopy in single cells. Annu. Rev. Cell Dev. Biol. 25, 301–327 (2009).
Nam, J.M., Nair, P.M., Neve, R.M., Gray, J.W. & Groves, J.T. A fluid membrane-based soluble ligand-display system for live-cell assays. Chembiochem 7, 436–40 (2006).
Nye, J.A. & Groves, J.T. Kinetic control of histidine-tagged protein surface density on supported lipid bilayers. Langmuir 24, 4145–4149 (2008).
Gureasko, J. et al. Membrane-dependent signal integration by the Ras activator Son of sevenless. Nat. Struct. Mol. Biol. 15, 452–461 (2008).
Axelrod, D., Koppel, D.E., Schlessinger, J., Elson, E. & Webb, W.W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 16, 1055–1069 (1976).
Groves, J.T., Parthasarathy, R. & Forstner, M.B. Fluorescence imaging of membrane dynamics. Annu. Rev. Biomed. Eng. 10, 311–338 (2008).
Kung, L.A., Kam, L., Hovis, J.S. & Boxer, S.G. Patterning hybrid surfaces of proteins and supported lipid bilayers. Langmuir 16, 6773–6776 (2000).
Groves, J.T., Ulman, N. & Boxer, S.G. Micropatterning fluid lipid bilayers on solid supports. Science 275, 651–653 (1997).
Cremer, P.S., Groves, J.T., Kung, L.A. & Boxer, S.G. Writing and erasing barriers to lateral mobility into fluid phospholipid bilayers. Langmuir 15, 3893–3896 (1999).
Gates, B.D., Xu, Q., Love, J.C., Wolfe, D.B. & Whitesides, G.M. Unconventional nanofabrication. Annu. Rev. Mater. Res. 34, 339–372 (2004).
Gonsalves, K.E., Thiyagarajan, M. & Dean, K.R. New resists for nanometer scale patterning by extreme ultraviolet lithography. J. Micro/Nanolithography, MEMS, and MOEMS 4, 029701 (2005).
Salaita, K., Wang, Y. & Mirkin, C.A. Applications of dip-pen nanolithography. Nat. Nano. 2, 145–155 (2007).
Salaita, K. et al. Massively parallel dip-pen nanolithography with 55 000-pen two-dimensional arrays. Angew. Chem. Int. Ed. Engl. 45, 7220–7223 (2006).
Salaita, K. et al. Sub-100 nm, centimeter-scale, parallel dip-pen nanolithography. Small 1, 940–945 (2005).
Tenchov, B.G., Yanev, T.K., Tihova, M.G. & Koynova, R.D. A probability concept about size distributions of sonicated lipid vesicles. Biochim. Biophys. Acta 816, 122–130 (1985).
Lasic, D.D. Liposomes in Gene Delivery (CRC Press, 1997).
Ollivon, M., Lesieur, S., Grabielle-Madelmont, C. & Paternostre, M. Vesicle reconstitution from lipid-detergent mixed micelles. Biochim. Biophys. Acta 1508, 34–50 (2000).
Richter, R.P., Him, J.L.K. & Brisson, A. Supported lipid membranes. Mater. Today 6, 32–37 (2003).
Ochsner, M. et al. Micro-well arrays for 3D shape control and high resolution analysis of single cells. Lab. Chip 7, 1074 (2007).
Schubert, D.W. & Dunkel, T. Spin coating from a molecular point of view: its concentration regimes, influence of molar mass and distribution. Mater. Res. Innovations 7, 314–321 (2003).
Qin, D., Xia, Y. & Whitesides, G.M. Soft lithography for micro- and nanoscale patterning. Nat. Protoc. 5, 491–502 (2010).
Cho, N., Frank, C.W., Kasemo, B. & Hook, F. Quartz crystal microbalance with dissipation monitoring of supported lipid bilayers on various substrates. Nat. Protoc. 5, 1096–1106 (2010).
Hallett, F.R., Watton, J. & Krygsman, P. Vesicle sizing: number distributions by dynamic light scattering. Biophys. J. 59, 357–362 (1991).
Gupta, K., Wasilik, M. & Williams, K.R. Etch rates for micromachining processing-part II. J. Microelectromechanical Syst. 12, 761–778.
Hovis, J.S. & Boxer, S.G. Patterning and composition arrays of supported lipid bilayers by microcontact printing. Langmuir 17, 3400–3405 (2001).
Yu, C., Parikh, A. & Groves, J. Direct patterning of membrane-derivatized colloids using in-situ uv-ozone photolithography. Adv. Mater. 17, 1477–1480 (2005).
Nagle, J.F. & Tristram-Nagle, S. Structure of lipid bilayers. Biochim. Biophys. Acta 1469, 159–195 (2000).
Galush, W.J., Nye, J.A. & Groves, J.T. Quantitative fluorescence microscopy using supported lipid bilayer standards. Biophys. J. 95, 2512–2519 (2008).
Misawa, Y., Li, Y., Rekosh, D. & Hammarskjold, M.L. Western blot analysis of sub-cellular fractionated samples using the Odyssey Infrared Imaging System. Nat. Protoc. doi:10.1038/nprot.2006.219 (2006).
Janes, P.W. et al. Adam meets Eph: An ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell 123, 291–304 (2005).
Walker-Daniels, J., Riese, D.J. & Kinch, M.S. c-Cbl-dependent EphA2 protein degradation is induced by ligand binding. Mol. Cancer Res. 1, 79–87 (2002).
Acknowledgements
We thank J.W. Gray and R.M. Neve for discussions that led to the use of supported membranes to study EphA2-ephrin-A1 signaling, and for providing the cells used in this work. We also thank N. Bayani for assistance in performing western blotting, A. Smoligovets and C.-H. Yu for performing transfection and imaging with EGFP-actin-expressing MDA-MB-231 cells, and A. Bershadsky for helpful discussions. This work was supported by the Director, Office of Science, Office of Basic Energy Sciences, Chemical Sciences, Geosciences and Biosciences Division (K.S., P.M.N.; hybrid synthetic-live cell interfaces) and Materials Sciences and Engineering Division (R.S.P.; supported membrane substrates) of the U.S. Department of Energy (DOE) under contract no. DE-AC02-05CH11231. Patterned substrate fabrication was performed, in part, at the Molecular Foundry, Lawrence Berkeley National Laboratory (LBNL), and was supported by the Office of Science, Office of Basic Energy Sciences, Scientific User Facilities Division of the U.S. DOE under contract no. DE-AC02-05CH11231. This work was also supported by the Laboratory Directed Research and Development Program of LBNL under U.S. DOE contract no. DE-AC02-05CH11231. Seed support for biomedical aspects of this work was provided by the U.S. Department of Defense Breast Cancer Research Program Concept Award BC076701 under U.S. Army Medical Research Acquisition Activity no. W81XWH-08-1-0677 with follow-on support provided by Award U54 CA143836 from the National Cancer Institute (NCI) beginning in 2009. K.S. acknowledges Oak Ridge National Laboratory's Center for Nanophase Materials Sciences, Scientific User Facilities Division, Office of Basic Energy Sciences, U.S. Department of Energy (CNMS2009-269). K.S. is also grateful to the Georgia Cancer Coalition (GCC) for a Cancer Research Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCI or the National Institutes of Health (NIH). The Regents of the University of California have filed a related patent application through LBNL.
Author information
Authors and Affiliations
Contributions
P.M.N. wrote the ImageJ software used to quantify metalloprotease recruitment to EphA2 clusters. P.M.N. and K.S. designed and performed all experiments described here. R.S.P. optimized and performed all E-beam lithography used to fabricate the patterned supported membranes. J.T.G. oversaw all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Methods 1
Calibration Calculator ImageJ macro. This analysis package first calculates an average total internal reflection fluorescence (TIRF) microscopy image from a series of images in a single channel. Background is calculated from the average intensity of a section of the images that is not illuminated in TIRF. Background is then subtracted from each pixel of the average image. (TXT 2 kb)
Supplementary Methods 2
Convert and Calibrate ImageJ macro. This analysis package separates microscopy data into three channels (bright field, fluorescence 1, fluorescence 2), subtracts background intensities from the two fluorescence channels, and calibrates for uneven TIRF illumination across a single image and between the two fluorescence channels. (TXT 2 kb)
Supplementary Methods 3
Cell Selector and Ratio Calculator ImageJ macro. This analysis package crops pre-defined user-selected areas (corresponding to cells) from a set of microscopy images and calculates the ratio of fluorescence intensities between two channels for each cell. (TXT 3 kb)
Supplementary Methods 4
Pearson Calculator ImageJ macro. This analysis package calculates the Pearson’s correlation coefficient between two fluorescence channels for single cells. (TXT 2 kb)
Supplementary Methods 5
SUV Calculator spreadsheet. This spreadsheet calculates the volumes of different lipid stock solutions to be mixed together to create a desired vesicle suspension in water. Input parameters are: lipid type, lipid molecular weight, stock solution concentrations (in mg/ml), desired final lipid composition (in mol % of each lipid type), desired final concentration of lipids in solution (in mg/ml), and desired final volume. Instructions for editing the spreadsheet are included in cell A5 of sheet 1. (XLS 75 kb)
Supplementary Manual
ImageJ analysis package readme. This text file contains detailed information regarding the use of supplementary ImageJ macros (Supplementary Methods 1-4), in terms of data collection formatting requirements and output file types. (TXT 3 kb)
Rights and permissions
About this article
Cite this article
Nair, P., Salaita, K., Petit, R. et al. Using patterned supported lipid membranes to investigate the role of receptor organization in intercellular signaling. Nat Protoc 6, 523–539 (2011). https://doi.org/10.1038/nprot.2011.302
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2011.302
This article is cited by
-
Dual-color DNA-PAINT single-particle tracking enables extended studies of membrane protein interactions
Nature Communications (2023)
-
Membrane anchoring facilitates colocalization of enzymes in plant cytochrome P450 redox systems
Communications Biology (2021)
-
Utility of TPP-manufactured biophysical restrictions to probe multiscale cellular dynamics
Bio-Design and Manufacturing (2021)
-
Solvent-assisted preparation of supported lipid bilayers
Nature Protocols (2019)
-
Resolving molecule-specific information in dynamic lipid membrane processes with multi-resonant infrared metasurfaces
Nature Communications (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.