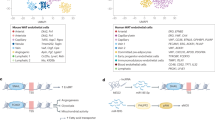
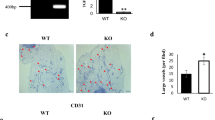
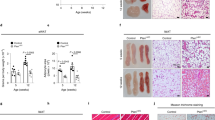
Abstract
Genetic and diet-induced rodent obesity models provide outstanding opportunities to study the role of angiogenesis and vascular remodeling in modulation of adipogenesis and obesity. In this study, we describe methods to quantitatively study adipose angiogenesis and vascular remodeling on the basis of immunohistochemical analyses. Fresh white adipose tissue or brown adipose tissue are prepared for whole mount, cryosectioned and paraffin-embedded samples, followed by staining with specific markers such as platelet endothelial cell adhesion molecule-1 (PECAM-1)/CD31, CD34, isolectin B4 or α-smooth muscle actin. Adipocytes are visualized by staining lipid droplets with 4,4-difluoro-4-bora-3a,4a-diaza-s-indacene-3-dodecanoic acid (BODIPY) 558/568 C12. This protocol may take 2–5 d to obtain results. In the view of the crucial roles of vasculature in modulation of adipogenesis and obesity, this protocol is valuable for studying the molecular mechanisms of angiogenesis in obese adipose tissues and for assessing the anti-obesity activity of angiogenesis modulators.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Carmeliet, P. & Jain, R.K. Angiogenesis in cancer and other diseases. Nature 407, 249–257 (2000).
Cao, Y. Angiogenesis and lymphangiogenesis in common diseases. Editorial. Curr. Mol. Med. 9, 928 (2009).
Lim, S.D. et al. Expression of the neural stem cell markers NG2 and L1 in human angiomyolipoma: are angiomyolipomas neoplasms of stem cells? Mol. Med. 13, 160–165 (2007).
Folkman, J. Angiogenesis: an organizing principle for drug discovery? Nat. Rev. Drug. Discov. 6, 273–286 (2007).
Cao, Y., Zhong, W. & Sun, Y. Improvement of antiangiogenic cancer therapy by understanding the mechanisms of angiogenic factor interplay and drug resistance. Semin. Cancer Biol. 19, 338–343 (2009).
Hurwitz, H. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350, 2335–2342 (2004).
Kerbel, R.S. Tumor angiogenesis. N. Engl. J. Med. 358, 2039–2049 (2008).
Rosenfeld, P.J. et al. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 355, 1419–1431 (2006).
Gragoudas, E.S., Adamis, A.P., Cunningham, E.T. Jr., Feinsod, M. & Guyer, D.R. Pegaptanib for neovascular age-related macular degeneration. N. Engl. J. Med. 351, 2805–2816 (2004).
Brakenhielm, E. et al. Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ. Res. 94, 1579–1588 (2004).
Cao, Y. Angiogenesis modulates adipogenesis and obesity. J. Clin. Invest. 117, 2362–2368 (2007).
Rupnick, M.A. et al. Adipose tissue mass can be regulated through the vasculature. Proc. Natl. Acad. Sci. USA 99, 10730–10735 (2002).
Xue, Y. et al. FOXC2 controls Ang-2 expression and modulates angiogenesis, vascular patterning, remodeling, and functions in adipose tissue. Proc. Natl. Acad. Sci. USA 105, 10167–10172 (2008).
Xue, Y. et al. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell. Metab. 9, 99–109 (2009).
Planat-Benard, V. et al. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation 109, 656–663 (2004).
Dobson, D.E. et al. 1-Butyryl-glycerol: a novel angiogenesis factor secreted by differentiating adipocytes. Cell 61, 223–230 (1990).
Brown, J.Q. et al. Quantitative optical spectroscopy: a robust tool for direct measurement of breast cancer vascular oxygenation and total hemoglobin content in vivo. Cancer Res. 69, 2919–2926 (2009).
Vineberg, A.M. et al. Myocardial revascularization by Omental graft without pedicle: experimental background and report on 25 cases followed 6 to 16 months. J. Thorac. Cardiovasc. Surg 49, 103–129 (1965).
Cao, R., Brakenhielm, E., Wahlestedt, C., Thyberg, J. & Cao, Y. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc. Natl. Acad. Sci. USA 98, 6390–6395 (2001).
Brakenhielm, E. et al. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc. Natl. Acad. Sci. USA 101, 2476–2481 (2004).
Bjorndahl, M. et al. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc. Natl. Acad. Sci. USA 102, 15593–15598 (2005).
Cao, R. et al. Hepatocyte growth factor is a lymphangiogenic factor with an indirect mechanism of action. Blood 107, 3531–3536 (2006).
Wicki, A. et al. Tumor invasion in the absence of epithelial-mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer. Cell. 9, 261–272 (2006).
Cho, C.H. et al. Angiogenic role of LYVE-1-positive macrophages in adipose tissue. Circ. Res. 100, e47–57 (2007).
Hedlund, E.M., Hosaka, K., Zhong, Z., Cao, R. & Cao, Y. Malignant cell-derived PlGF promotes normalization and remodeling of the tumor vasculature. Proc. Natl. Acad. Sci. USA. 106, 17505–17510 (2009).
Acknowledgements
Yihai Cao's laboratory is supported through research grants from the Swedish Research Council, the Swedish Cancer Foundation, the Karolinska Institute Foundation, the Karolinska Institute Distinguished Professor Award, the European Union Integrated Project of Metoxia (Project no. 222741) and the European Research Council (ERC) advanced grant ANGIOFAT (Project no. 250021).
Author information
Authors and Affiliations
Contributions
Y.C. designed the study. Y.X. and S.L. performed and analyzed experiments. E.B. contributed to the development of these protocols. Y.X., S.L. and Y.C. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Xue, Y., Lim, S., Bråkenhielm, E. et al. Adipose angiogenesis: quantitative methods to study microvessel growth, regression and remodeling in vivo. Nat Protoc 5, 912–920 (2010). https://doi.org/10.1038/nprot.2010.46
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2010.46
This article is cited by
-
Small extracellular vesicles from human adipose-derived mesenchymal stromal cells: a potential promoter of fat graft survival
Stem Cell Research & Therapy (2021)
-
Lymphatic vessels in human adipose tissue
Cell and Tissue Research (2020)
-
A direct tissue-grafting approach to increasing endogenous brown fat
Scientific Reports (2018)
-
Carboxytherapy-Induced Fat loss is Associated with VEGF-Mediated Vascularization
Aesthetic Plastic Surgery (2018)
-
Histomorphometric analyses of human adipose tissues using intact, flash-frozen samples
Histochemistry and Cell Biology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.