Abstract
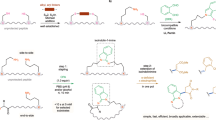
This protocol describes a method for synthesizing peptide macrocycles from linear peptide precursors, isocyanides and aziridine aldehydes. The effects of the reaction components on the efficiency of the process are discussed. Macrocyclization is exemplified by the preparation of a nine-membered ring peptide macrocycle. The product is further functionalized by nucleophilic opening of the aziridine ring with a fluorescent thiol. This transformation constitutes a useful late-stage functionalization of a macrocyclic peptide molecule. The experimental section describes the selection of the required starting materials, and the preparation of a representative aziridine-2-carboxaldehyde dimer. The synthesis and isolation of the peptide macrocycle can be accomplished in 6 h, and the ring-opening requires approximately 6–8 h. The aziridine-2-carboxaldehyde reagent is commercially available or can be synthesized from readily available starting materials in approximately 4 d. The strategy described is not limited to the specific peptide, isocyanide, aziridine aldehyde or nucleophile used in the representative synthesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Robinson, J.A. β-Hairpin peptidomimetics: design, structures and biological activities. Acc. Chem. Res. 41, 1278–1288 (2008).
Chatterjee, J., Gilon, C., Hoffman, A. & Kessler, H. N-Methylation of peptides: a new perspective in medicinal chemistry. Acc. Chem. Res. 41, 1331–1342 (2008).
Geyer, A., Müller, G. & Kessler, H. Conformational analysis of a cyclic RGD peptide containing a ψ[CH2–NH] bond: a positional shift in backbone structure caused by a single dipeptide mimetic. J. Am. Chem. Soc. 116, 7735–7743 (1994).
Driver, R.W., Hoang, H.N., Abbenante, G. & Fairlie, D.P. A cyclic β-strand tripeptide with an α-helix like CD spectrum. Org. Lett. 11, 3092–3095 (2009).
Rezai, T. et al. Testing the conformational hypothesis of passive membrane permeability using synthetic cyclic peptide diastereomers. J. Am. Chem. Soc. 128, 2510–2511 (2006).
Hess, S. et al. Backbone cyclic peptidomimetic melanocortin-4 receptor agonist as a novel orally administrated drug lead for treating obesity. J. Med. Chem. 51, 1026–1034 (2008).
Fletcher, J.M. et al. Design of a conformationally defined and proteolytically stable circular mimetic of brain-derived neurotrophic factor. J. Biol. Chem. 283, 33375–3383 (2008).
Tyndall, J.D.A., Nall, T. & Fairlie, D.P. Proteases universally recognize beta strands in their active sites. Chem. Rev. 105, 973–999 (2005).
Gilon, C., Mang, C., Lohof, E., Friedler, A. & Kessler, H. 6.8 Synthesis of cyclic peptides. in Synthesis of Peptides and Peptidomimetics Vol. E 22b (ed. Goodman, M.) 461–542 (Thieme, New York, USA, 2004).
Hardy, P.M., Kenner, G.W. & Sheppard, R.C. Peptides—XIII: effects of configuration on dielectric increments and cyclization of some simple peptides. Tetrahedron 19, 95–105 (1963).
Dale, J. Conformational aspects of many-membered rings. Angew. Chem. Int. Ed. Engl. 5, 1000–1021 (1966).
Benoiton, N.L., Lee, Y.C., Steinaur, R. & Chen, F.M.F. Studies on sensitivity to racemization of activated residues in couplings of N-benzyloxycarbonyldipeptides. Int. J. Pept. Protein Res. 40, 559–566 (1992).
Tamaki, M., Akabori, S. & Muramatsu, I. Biomimetic synthesis of gramicidin S. Direct formation of the antibiotic from pentapeptide active esters having no protecting group on the side chain of the Orn residue. J. Am. Chem. Soc. 115, 10492–10496 (1993).
Kessler, H. & Haase, B. Cyclic hexapeptides derived from the human thymopoietin III. Int. J. Pept. Protein Res. 39, 36–40 (1992).
Schmidt, U. & Langner, J. Cyclotetrapeptides and cyclopentapeptides: occurrence and synthesis. J. Pept. Res. 49, 67–73 (1997).
Mitova, M., Popov, S. & De Rosa, S. Cyclic peptides from a Ruegeria strain of bacteria associated with the sponge Suberites domuncula. J. Nat. Prod. 67, 1178–1181 (2004).
Bean, J.W., Kopple, K.D. & Peishoff, C.E. Conformational analysis of cyclic hexapeptides containing the D-Pro-L-Pro sequence to fix β-turn positions. J. Am. Chem. Soc. 114, 5328–5334 (1992).
Kurath, P. 116. Preparation and antimicrobial activity of enantio-[1-valine]malformin. Helv. Chim. Acta. 59, 1127–1132 (1976).
Brady, S.F. et al. Large-scale synthesis of a cyclic hexapeptide analogue of somatostatin. J. Org. Chem. 52, 764–769 (1987).
Schmidt, U., Lieberknecht, A., Griesser, H. & Talbiersky, J. Synthesis of peptide alkaloids. 5. New methods for synthesis of ansa peptides. Amino acids and peptides. 34. J. Org. Chem. 47, 3261–3264 (1982).
Carpino, L. 1-Hydroxy-7-azabenzotriazole. An efficient peptide coupling additive. J. Am. Chem. Soc. 115, 4397–4398 (1993).
Carpino, L.A., El-Faham, A. & Albericio, F. Racemization studies during solid-phase peptide synthesis using azabenzotriazole-based coupling reagents. Tetrahedron Lett. 35, 2279–2282 (1994).
Tavassoli, A. & Benkovic, S.J. Split-intein mediated circular ligation used in the synthesis of cyclic peptide libraries in E. coli. Nat. Protoc. 2, 1126–1133 (2007).
Lambert, J.N., Mitchell, J.P. & Roberts, K.D. The synthesis of cyclic peptides. J. Chem. Soc. Perkin Trans. 1, 471–484 (2001).
Blackwell, H.E. & Grubbs, R.H. Highly efficient synthesis of covalently cross-linked peptide helices by ring-closing metathesis. Angew. Chem. Int. Ed. Engl. 37, 3281–3284 (1998).
Schafmeister, C.E., Po, J. & Verdine, G.L. An all-hydrocarbon cross-linking system for enhancing the helicity and metabolic stability of peptides. J. Am. Chem. Soc. 122, 5891–5892 (2000).
Angell, Y. & Burgess, K. Ring closure to β-turn mimics via copper-catalyzed azide/alkyne cycloadditions. J. Org. Chem. 70, 9595–9598 (2005).
Hili, R., Rai, V. & Yudin, A.K. Macrocyclization of linear peptides enabled by amphoteric molecules. J. Am. Chem. Soc. 132, 2889–2891 (2010).
Hili, R. & Yudin, A.K. Amphoteric amino aldehydes reroute the aza-Michael reaction. J. Am. Chem. Soc. 131, 16404–16406 (2009).
Baktharaman, S., Hili, R. & Yudin, A.K. Amino carbonyl compounds in organic synthesis. Aldrichimica Acta 41, 109–119 (2008).
Hili, R. & Yudin, A.K. Readily available unprotected amino aldehydes. J. Am. Chem. Soc. 128, 14772–14773 (2006).
Marcaccini, S. & Torroba, T. The use of the Ugi four-component condensation. Nat. Protoc. 2, 632–639 (2007).
Dömling, A. & Ugi, I. Multicomponent reactions with isocyanides. Angew. Chem. Int. Ed. Engl. 39, 3168–3210 (2000).
Failli, A., Immer, H. & Götz, M. The synthesis of cyclic peptide by the four component condensation (4 CC). Can. J. Chem. 57, 3257–3261 (1979).
Galoni, D., Ide, N.D., van der Donk, W.A. & Gin, D.Y. Aziridine-2-carboxylic acid-containing, peptides: application to solution- and solid-phase convergent site-selective peptide modification. J. Am. Chem. Soc. 127, 7359–7369 (2005).
Moss, T.A., Alba, A., Hepworth, D. & Dixon, D.J. Efficient base catalyzed alkylation reactions with aziridine electrophiles. Chem. Commun. 2474–2476 (2008).
Ulrike, R. et al. Nucleophilic ring-opening of activated aziridines: a one-step method for labeling biomolecules with fluorine-18. J. Fluorine Chem. 130, 902–912 (2009).
Conti, F., Lucente, G., Romeo, A. & Zanotti, G. Cyclol-formation from tripeptide systems and structure assignment by carbon-13 nuclear magnetic resonance. Int. J. Pept. Protein Res. 5, 353–357 (1973).
Skorna, G. & Ugi, I. Isocyanide synthesis with diphosgene. Angew. Chem. Int. Ed. Engl. 16, 259–260 (1977).
Obrecht, R., Herrmann, R. & Ugi, I. Isocyanide synthesis with phosphoryl chloride and diisopropylamine. Synthesis 400–402 (1985).
Zhou, P., Chen, B.-C. & Davis, F.A. Asymmetric syntheses with aziridinecarboxylate and aziridinephosphonate building blocks. in Aziridines and Epoxides in Organic Synthesis. (ed. Yudin, A.K.) 73–115 (Wiley-VCH, Weinheim, Germany, 2006).
Author information
Authors and Affiliations
Contributions
B.R., V.R. and R.H. carried out the syntheses and characterization. A.Y. designed the experiments and supervised the overall project. All authors co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Rotstein, B., Rai, V., Hili, R. et al. Synthesis of peptide macrocycles using unprotected amino aldehydes. Nat Protoc 5, 1813–1822 (2010). https://doi.org/10.1038/nprot.2010.127
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2010.127
This article is cited by
-
Convergent synthesis of aminomethylene peptidomimetics
Nature Protocols (2012)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.