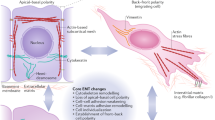
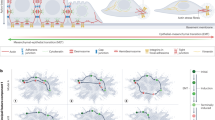
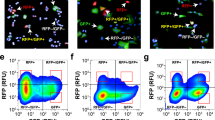
Abstract
Here we describe several methods for the characterization of epithelial–mesenchymal transition (EMT) at the cellular, molecular and behavioral level. This protocol describes both in vitro and in vivo approaches designed to analyze different features that when taken together permit the characterization of cells undergoing transient or stable EMT. We define straightforward methods for phenotypical, cellular and transcriptional characterization of EMT in vitro in monolayer cultures. The procedure also presents technical details for the generation of in vitro three-dimensional (3D) cultures analyzing cell phenotype and behavior during the EMT process. In addition, we describe xenotransplantation techniques to graft 3D cell cultures into mice to study in vivo invasion in a physiological-like environment. Finally, the protocol describes the analysis of selected EMT markers from experimental and human tumor samples. This series of methods can be applied to the study of EMT under various experimental and biological situations. Once the methodology is established, the time required to complete the protocol may vary from 3 to 4 weeks (monolayer cultures) and up to 6–8 weeks if including 3D cultures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Hay, E.D. An overview of epithelio-mesenchymal transformation. Acta. Anat. (Basel) 154, 8–20 (1995).
Thiery, J.P. Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2, 442–454 (2002).
Huber, M.A., Kraut, N. & Beug, H. Molecular requirements for epithelial–mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 17, 548–558 (2005).
Peinado, H., Olmeda, D. & Cano, A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat. Rev. Cancer 7, 415–428 (2007).
Barrallo-Gimeno, A. & Nieto, M.A. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 132, 3151–3161 (2005).
Moreno-Bueno, G., Portillo, F. & Cano, A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene 27, 6958–6969 (2008).
Moreno-Bueno, G. et al. Genetic profiling of epithelial cells expressing E-cadherin repressors reveals a distinct role for Snail, Slug, and E47 factors in epithelial–mesenchymal transition. Cancer Res. 66, 9543–9556 (2006).
De Craene, B. et al. The transcription factor snail induces tumor cell invasion through modulation of the epithelial cell differentiation program. Cancer Res. 65, 6237–6244 (2005).
Gupta, G.P. & Massagué, J. Cancer metastasis: building a framework. Cell 127, 679–695 (2006).
Yang, J. & Weinberg, R.A. Epithelial–mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell 14, 818–829 (2008).
Christofori, G. New signals from the invasive front. Nature 441, 444–450 (2006).
Nieto, M.A. Epithelial–mesenchymal transitions in development and disease: old views and new perspectives. Int. J. Dev. Biol. doi:10.1387/ijdb.072410mn (2008).
Mani, S.A. et al. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 (2008).
Hugo, H. et al. Epithelial–mesenchymal and mesenchymal–epithelial transitions in carcinoma progression. J. Cell. Physiol. 213, 374–383 (2007).
Polyak, K. & Weinberg, R.A. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat. Rev. Cancer 9, 265–273 (2009).
Christiansen, J.J. & Rajasekaran, A.K. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 66, 8319–8326 (2006).
Scheel, C., Onder, T., Karnoub, A. & Weinberg, R.A. Adaptation versus selection: the origins of metastatic behavior. Cancer Res. 67, 11476–11479; discussion: 11479–11480 (2007).
Thiery, J.P. & Sleeman, J.P. Complex networks orchestrate epithelial–mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 7, 131–142 (2006).
de Craene, V., van Roy, F. & Berx, G. Unraveling signalling cascades for the Snail family of transcription factors. Cell. Signal 17, 535–547 (2005).
Moustakas, A. & Heldin, C.H. Signaling networks guiding epithelial–mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 98, 1512–1520 (2007).
Zavadil, J. & Böttinger, E.P. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene 24, 5764–5774 (2005).
Massagué, J. TGF in cancer. Cell 134, 215–230 (2008).
Gort, E.H. et al. Hypoxic regulation of metastasis via hypoxia-inducible factors. Curr. Mol. Med. 8, 60–67 (2008).
Nieto, M.A. The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 3, 155–166 (2002).
Acloque, H., Adams, M.S., Fishwick, K., Bronner-Fraser, M. & Nieto, M.A. Epithelial–mesenchymal transitions: the importance of changing cell state in development and disease. J. Clin. Invest. 119, 1438–1449 (2009).
Guaita, S. et al. Snail induction of epithelial to mesenchymal transition in tumor cells is accompanied by MUC1 repression and ZEB1 expression. J. Biol. Chem. 277, 39209–39216 (2002).
Kang, Y. & Massagué, J. Epithelial to mesenchymal transition: twist in development and metastasis. Cell 6, 277–279 (2004).
Cano, A. et al. The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2, 76–83 (2000).
Perez-Moreno, M.A. et al. A new role for E12/E47 in the repression of E-cadherin expression and epithelial–mesenchymal transitions. J. Biol. Chem. 276, 27424–27431 (2001).
Bolós, V. et al. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J. Cell Sci. 116, 499–511 (2003).
Peinado, H., Quintanilla, M. & Cano, A. Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J. Biol. Chem. 278, 21113–21123 (2003).
Peinado, H. et al. Snail and E47 repressors of E-cadherin induce distinct invasive and angiogenic properties in vivo . J. Cell Sci. 117, 2827–2839 (2004).
Peinado, H. et al. A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. EMBO J. 24, 3446–3458 (2005).
Sobrado, V.R. et al. The class I bHLH factors E2-2A and E2-2B regulate EMT. J. Cell Sci. 122, 1014–1024 (2009).
Peinado, H. et al. Lysyl oxidase-like 2 as a new poor prognosis marker of squamous cell carcinomas. Cancer Res. 68, 4541–4550 (2008).
Sarrio, D. et al. Epithelial–mesenchymal transition in breast cancer relates to the basal-like phenotype. Cancer Res. 68, 989–997 (2008).
Miller, D.S. et al. Manipulating cell migration and proliferation with a light-activated polypeptide. Chembiochem 10, 577–584 (2009).
Sarrió, D. et al. Functional characterization of E- and P-cadherin in invasive breast cancer cells. BMC Cancer 9, 74 (2009).
Maas-Szabowski, N., Fusenig, N.E. & Stark, H.J. Experimental models to analyze differentiation functions of cultured keratinocytes in vitro and in vivo . Methods Mol. Biol. 289, 47–60 (2005).
Batlle, E. et al. The transcription factor Snail is a repressor of E-cadherin gene expression in epithelial tumor cells. Nat. Cell Biol. 2, 84–89 (2000).
Yang, J. et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117, 927–939 (2004).
Comijn, J. et al. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol. Cell 7, 1267–1278 (2001).
Eger, A. et al. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene 24, 2375–2385 (2005).
Mani, S.A. et al. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc. Natl. Acad. Sci. USA 104, 10069–10074 (2007).
Hartwell, K.A. et al. The Spemann organizer gene, Goosecoid, promotes tumor metastasis. Proc. Natl. Acad. Sci. USA 103, 18969–18974 (2006).
Thuault, S. et al. Transforming growth factor-beta employs HMGA2 to elicit epithelial–mesenchymal transition. J. Cell Biol. 174, 175–183 (2006).
Schramek, H. et al. Loss of active MEK1-ERK1/2 restores epithelial phenotype and morphogenesis in transdifferentiated MDCK cells. Am. J. Physiol. Cell Physiol. 285, C652–C661 (2003).
Montesano, R., Soulié, P., Eble, J.A. & Carrozzino, F. Tumour necrosis factor alpha confers an invasive, transformed phenotype on mammary epithelial cells. J. Cell Sci. 118, 3487–3500 (2005).
Gal, A. et al. Sustained TGF beta exposure suppresses Smad and non-Smad signalling in mammary epithelial cells, leading to EMT and inhibition of growth arrest and apoptosis. Oncogene 27, 1218–1230 (2008).
Waerner, T. et al. ILEI: a cytokine essential for EMT, tumor formation, and late events in metastasis in epithelial cells. Cancer Cell 10, 227–339 (2006).
Sabeh, F. et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J. Cell Biol. 167, 769–781 (2004).
Rowe, R.G. et al. Mesenchymal cells reactivate Snail1 expression to drive three-dimensional invasion programs. J. Cell Biol. 184, 399–408 (2009).
Medici, D., Hay, E.D. & Goodenough, D.A. Cooperation between snail and LEF-1 transcription factors is essential for TGF-beta1-induced epithelial–mesenchymal transition. Mol. Biol. Cell 17, 1871–1879 (2006).
Ohkubo, T. & Ozawa, M.J. The transcription factor Snail downregulates the tight junction components independently of E-cadherin downregulation. Cell Sci. 117, 1675–1685 (2004).
Savagner, P., Yamad, K.M. & Thiery, J.P. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial–mesenchymal transition. J. Cell Biol. 137, 1403–1419 (1997).
Zhou, B.P. et al. Dual regulation of Snail by GSK-3beta-mediated phos-phorylation in control of epithelial–mesenchymal transition. Nat. Cell Biol. 6, 931–940 (2004).
Arima, Y. et al. Rb depletion results in deregulation of E-cadherin and induction of cellular phenotypic changes that are characteristic of the epithelial-to-mesenchymal transition. Cancer Res. 68, 5104–5112 (2008).
Guan, F., Handa, K. & Hakomori, S.I. Specific glycosphingolipids mediate epithelial-to-mesenchymal transition of human and mouse epithelial cell lines. Proc. Natl. Acad. Sci. USA 106, 7461–7466 (2009).
Lu, Z., Ghosh, S., Wang, Z. & Hunter, T. Hepatocyte growth factor induces cell scattering through MAPK/Egr-1-mediated upregulation of Snail. Cancer Cell 4, 499–515 (2003).
Grotegut, S., von Schweinitz, D., Christofori, G. & Lehembre, F. Hepatocyte growth factor induces cell scattering through MAPK/Egr-1-mediated upregulation of Snail. EMBO J. 25, 3534–3545 (2006).
Gotzmann, J. et al. A crucial function of PDGF in TGF-mediated cancer progression of hepatocytes. Oncogene 25, 3170–3185 (2006).
Lien, H.C. et al. Molecular signatures of metaplastic carcinoma of the breast by large-scale transcriptional profiling: identification of genes potentially related to epithelial–mesenchymal transition. Oncogene 26, 7859–7871 (2007).
Tomita, K. et al. Cadherin switching in human prostate cancer progression. Cancer Res. 60, 3650–3654 (2000).
Locascio, A. et al. Biological potential of a functional human SNAIL retrogene. J. Biol. Chem. 277, 38803–38809 (2002).
Olmeda, D. et al. SNAI1 is required for tumor growth and lymph node metastasis of human breast carcinoma MDA-MB-231 cells. Cancer Res. 67, 11721–11731 (2007).
Olmeda, D. et al. Snai1 and Snai2 collaborate on tumor growth and metastasis properties of mouse skin carcinoma cell lines. Oncogene 27, 4690–4701 (2008).
Brown, K. et al. v-ras genes from Harvey and BALB murine sarcoma viruses can act as initiators of two-stage mouse skin carcinogenesis. Cell 46, 447–456 (1986).
Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970).
Janda, E. et al. Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J. Cell Biol. 156, 299–313 (2002).
Martin-Villar, E. et al. Podoplanin binds ERM proteins to activate RhoA and promote epithelial–mesenchymal transition. J. Cell Sci. 119, 4541–4553 (2006).
Shirakihara, T., Saitoh, M. & Miyazono, K. Differential regulation of epithelial and mesenchymal markers by deltaEF1 proteins in epithelial mesenchymal transition induced by TGF-beta. Mol. Biol. Cell 18, 3533–3544 (2007).
Behrens, J., Löwrick, O., Klein-Hitpass, L. & Birchmeier, W. The E-cadherin promoter: functional analysis of a G.C-rich region and an epithelial cell-specific palindromic regulatory element. Proc. Natl. Acad. Sci. USA 88, 11495–11499 (1991).
Shirayoshi, Y., Okada, T.S. & Takeichi, M. N-linked oligosaccharides are not involved in the function of a cell-cell binding glycoprotein E-cadherin. Cell Struct. Funct. 11, 245–252 (1986).
Acknowledgements
We are grateful to A. Montes and R. Díaz-Uriarte for technical assistance in generation of stable transfectants and in microarray data analysis, respectively, and to S. Lavotshkin for his friendly help with editorial assistance. This study has been supported by grant nos. SAF2004-00361, SAF2007-63051, SAF2007-63075 and Consolider-Ingenio 2010 CSD2007-00017 (MICIN, Spain), by MRTN 2004-005428 (European Commission) and by FMM07 (Fundación Mutua Madrileña).
Author information
Authors and Affiliations
Contributions
G.M.-B. was responsible for design and performance of microarray profiling studies including the statistical analysis, performed IHC analysis on tissue arrays and promoter assays, designed and carried out the Supplementary Methods, Figures 2, 4 and 8, and Tables 1, 2 and 7 and drafted this paper; H.P. was responsible for the design and analysis of 3D cultures (in vitro and in vivo), performed immunofluorescence analysis in monolayer and 3D cultures, designed and drew Box 2, Figures 6 and 7, and Table 3, collaborated in Figure 3 and drafted this paper; P.M. was responsible for the wound healing assays and collaborated in the IF analysis in monolayer cultures, designed and drew Box 1, Figures 1, 2 and 5, and Table 4 and drafted this paper; D.O. was responsible for the RT-PCR and qPCR analysis and for the design of species-specific primers (Table 5) and specific shRNAs probes; E.C. participated in the characterization of MDCK-E47 cell system at protein, mRNA and promoter level, collaborated in microarray analysis and drew Figures 3 and 4; V.S. collaborated with HP in the optimization of 3D cultures (in vitro and in vivo) and performed experiments described in Figure 7; J.P. performed, analyzed and interpreted the tissue arrays, and collaborated in drawing Figure 8; F.P. drew Tables 1, 2, 4, 5 and 6 and drafted this paper; A.C. coordinated the writing of this paper and together with F.P. was responsible for the final draft of this paper.
Corresponding author
Supplementary information
Supplementary Methods
Selection of EMT genes after gene profiling analysis (PDF 121 kb)
Rights and permissions
About this article
Cite this article
Moreno-Bueno, G., Peinado, H., Molina, P. et al. The morphological and molecular features of the epithelial-to-mesenchymal transition. Nat Protoc 4, 1591–1613 (2009). https://doi.org/10.1038/nprot.2009.152
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2009.152
This article is cited by
-
Brevinin-2R: Antimicrobial Peptide with Cytotoxic and Apoptogenic Activity Against Daunorubicin Resistant Gastric Cancer Cells
Pharmaceutical Chemistry Journal (2023)
-
Chronic Leptin Treatment Induces Epithelial-Mesenchymal Transition in MCF10A Mammary Epithelial Cells
Journal of Mammary Gland Biology and Neoplasia (2022)
-
Fluid flow exposure promotes epithelial-to-mesenchymal transition and adhesion of breast cancer cells to endothelial cells
Breast Cancer Research (2021)
-
RETRACTED ARTICLE: Long non-coding RNA LINC00525 regulates the proliferation and epithelial to mesenchymal transition of human glioma cells by sponging miR-338-3p
AMB Express (2020)
-
The role of epithelial–mesenchymal transition (EMT)-associated genes during gonadogenesis of albino rat
The Journal of Basic and Applied Zoology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.