Abstract
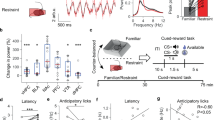
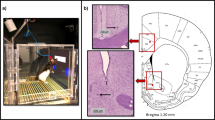
It has become increasingly important to assess mood states in laboratory animals. Tests that reflect reward, reduced ability to experience reward (anhedonia) and aversion (dysphoria) are in high demand because many psychiatric conditions that are currently intractable in humans (e.g., major depression, bipolar disorder, addiction) are characterized by dysregulated motivation. Intracranial self-stimulation (ICSS) can be utilized in rodents (rats, mice) to understand how pharmacological or molecular manipulations affect the function of brain reward systems. Although many different methodologies are possible, we will describe in this protocol the use of medial forebrain bundle (MFB) stimulation together with the 'curve-shift' variant of analysis. This combination is particularly powerful because it produces a highly reliable behavioral output that enables clear distinctions between the treatment effects on motivation and the treatment effects on the capability to perform the task.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Liebman, J.M. Discriminating between reward and performance: a critical review of intracranial self-stimulation methodology. Neurosci. Biobehav. Rev. 9, 45–72 (1983).
Bielajew, C. & Shizgal, P. Evidence implicating descending fibers in self-stimulation of the medial forebrain bundle. J. Neurosci. 6, 919–929 (1986).
Yeomans, J.S. et al. Brain-stimulation reward thresholds raised by an antisense oligonucleotide for the M5 muscarinic receptor infused near dopamine cells. J. Neurosci. 20, 8861–8867 (2000).
Carlezon, W.A. Jr. & Wise, R.A. Microinjections of phencyclidine (PCP) and related drugs into nucleus accumbens shell potentiate brain stimulation reward. Psychopharmacology (Berl) 128, 413–420 (1996).
Todtenkopf, M.S. et al. Brain reward regulated by glutamate receptor subunits in the nucleus accumbens shell. J. Neurosci. 26, 11665–11669 (2006).
Routtenberg, A. & Lindy, J. Effects of availability of rewarding septal and hypothalamic stimulation on bar pressing for food under conditions of deprivation. J. Comp. Physiol. Psychol. 60, 150–161 (1965).
Carlisle, H.J. & Snyder, E. The interaction of hypothalamic self-stimulation and temperature regulation. Experientia 26, 1092–1093 (1970).
Stewart, J. & Wise, R.A. Reinstatement of heroin self-administration habits: morphine prompts and naltrexone discourages renewed responding after extinction. Psychopharmacology (Berl) 108, 79–84 (1992).
Markou, A., Hauger, R.L. & Koob, G.F. Desmethylimipramine attenuates cocaine withdrawal in rats. Psychopharmacology (Berl) 109, 305–314 (1992).
Todtenkopf, M.S., Marcus, J.F., Portoghese, P.S. & Carlezon, W.A. Jr. Effects of κ-opioid ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 172, 463–470 (2004).
Carlezon, W.A. Jr. et al. Depressive-like effects of the κ-opioid receptor agonist Salvinorin A on behavior and neurochemistry in rats.. J. Pharmacol. Exp. Ther. 316, 440–447 (2006).
Gallistel, C.R., Shizgal, P. & Yeomans, J.S. A portrait of the substrate for self-stimulation. Psychol. Rev. 88, 228–273 (1981).
Yeomans, J.S. Principles of brain stimulation (Oxford University Press, New York, 1980).
Gallistel, C.R. & Freyd, G. Quantitative determination of the effects of catecholaminergic agonists and antagonists on the rewarding efficacy of brain stimulation. Pharmacol. Biochem. Behav. 26, 731–741 (1987).
Esposito, R.U. & Kornetsky, C. Morphine lowering of self-stimulation thresholds: lack of tolerance with long-term administration. Science 195, 189–191 (1977).
Gardner, E.L. et al. Facilitation of brain stimulation reward by Δ9-tetrahydrocannabinol. Psychopharmacology (Berl) 96, 142–144 (1988).
Miliaressis, E., Rompré, P.P. & Durivage, A. Psychophysical method for mapping behavioral substrates using a moveable electrode. Brain Res. Bull. 8, 693–701 (1982).
Wise, R.A. Addictive drugs and brain stimulation reward. Annu. Rev. Neurosci. 19, 319–340 (1996).
Barr, A.M., Markou, A. & Phillips, A.G. A 'crash' course on psychostimulant withdrawal as a model of depression. Trends Pharmacol. Sci. 23, 475–482 (2003).
Goussakov, I. et al. LTP in the amygdala during cocaine withdrawal. Eur. J. Neurosci. 23, 239–250 (2006).
Tomasiewicz, H.C., Mague, S.D., Cohen, B.M. & Carlezon, W.A. Jr. Behavioral effects of short-term administration of lithium and valproic acid in rats. Brain Res. 1093, 83–94 (2006).
Carlezon, W.A. Jr. & Wise, R.A. Phencyclidine-induced potentiation of brain stimulation reward: acute effects are not altered by repeated administration. Psychopharmacology (Berl) 111, 402–408 (1993).
Liu, J. & Schulteis, G. Brain reward deficits accompany naloxone-precipitated withdrawal from acute opioid dependence. Pharmacol. Biochem. Behav. 79, 101–108 (2004).
Arvanitogiannis, A., Riscaldino, L. & Shizgal, P. Effects of NMDA lesions of the medial basal forebrain on LH and VTA self-stimulation. Physiol. Behav. 65, 805–810 (1999).
Collins, R.J., Weeks, J.R., Cooper, M.M., Good, P.I. & Russell, R.R. Prediction of abuse liability of drugs using IV self-administration by rats. Psychopharmacology 82, 6–13 (1984).
Roybal, K. et al. Mania-like behavior induced by disruption of CLOCK function. Proc. Natl. Acad. Sci. USA 104, 6406–6411 (2007).
Elmer, G.I. et al. Brain stimulation and morphine reward deficits in dopamine D2 receptor-deficient mice. Psychopharmacology (Berl) 182, 33–44 (2005).
Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates 2nd edn. (Academic Press, San Diego, CA, 1986).
Paxinos, G. & Franklin, K.B.J. The Mouse Brain in Stereotaxic Coordinates 2nd edn. (Academic Press, San Diego, CA, 2001).
Gilliss, B., Malanga, C.J., Pieper, J.O. & Carlezon, W.A. Jr. Cocaine and SKF-82958 potentiate brain stimulation reward in Swiss-Webster mice. Psychopharmacology (Berl) 163, 238–248 (2002).
Carlezon, W.A. Jr. et al. Repeated exposure to rewarding brain stimulation downregulates GluR1 expression in the ventral tegmental area. Neuropsychopharmacology 25, 234–241 (2001).
Mague, S.D., Andersen, S.L. & Carlezon, W.A. Jr. Early developmental exposure to methylphenidate reduces cocaine-induced potentiation of brain stimulation reward in rats. Biol. Psychiatry 57, 120–125 (2005).
Bolaños, C.A., Barrot, M., Berton, O., Wallace-Black, D. & Nestler, E.J. Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol. Psychiatry 54, 1317–1329 (2003).
Acknowledgements
The authors were supported in part by grants from the National Institutes of Health (DA012736 and MH063266 to W.A.C.; DA023094 to E.H.C.) while writing this protocol.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
WAC discloses that his group received financial compensation (an individual registration for the Society for Neuroscience conference) from a manufacturer of ICSS equipment (Med Associates) in exchange for a description of technical procedures for its website. WAC is also a member of the scientific advisory board for a company (Myneurolab.com) that sells products described in this protocol.
Supplementary information
Rights and permissions
About this article
Cite this article
Carlezon, W., Chartoff, E. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc 2, 2987–2995 (2007). https://doi.org/10.1038/nprot.2007.441
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.441
This article is cited by
-
Advancing preclinical chronic stress models to promote therapeutic discovery for human stress disorders
Neuropsychopharmacology (2024)
-
Early life stress in male mice blunts responsiveness in a translationally-relevant reward task
Neuropsychopharmacology (2023)
-
Nanoassemblies from the aqueous extract of roasted coffee beans modulate the behavioral and molecular effects of smoking withdrawal–induced anxiety in female rats
Drug Delivery and Translational Research (2023)
-
Rate of onset of dopamine transporter inhibitors assessed with intracranial self-stimulation and in vivo dopamine photometry in rats
Psychopharmacology (2023)
-
Reactivating hippocampal-mediated memories during reconsolidation to disrupt fear
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.