Abstract
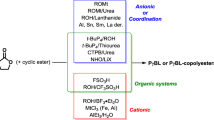
This protocol describes the synthesis of poly(L-lactide) by ring-opening polymerization of L-lactide using tin(II) 2-ethylhexanoate catalyst as well as the synthesis of polyglycolide by ring-opening polymerization of glycolide. Ring-opening polymerization of cyclic diesters synthesized from α-hydroxycarboxylic acids gives high-molecular-weight polyester in high yield. Tin(II) 2-ethylhexanoate catalyst is the most common catalyst for ring-opening polymerization of diesters owing to its high reactivity and low toxicity. Purity of monomers and the amount of water and alcohol in the reaction system are significant factors for increasing molecular weight and conversion of polyesters. The molecular weight of the polyesters is also dependent on reaction temperature and reaction time. This protocol can be completed in 3 d for the synthesis of poly(L-lactide) and 2 d for the synthesis of polyglycolide.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jacobsen, S., Degee, Ph., Fritz, H.G., Dubois, Ph & Jerome, R. Polylactide (PLA)—a new way of production. Polym. Eng. Sci. 39, 1311–1319 (1999).
Ikada, Y. & Tsuji, H. Biodegradable polyesters for medical and ecological applications. Macromol. Rapid Commun. 21, 117–132 (2000).
Athanasiou, K.A., Niederauer, G.G. & Agrawal, C.M. Sterilization, toxicity, biocompatibility and clinical applications of polylactic acid/polyglycolic acid copolymers. Biomaterials 17, 93–102 (1996).
Yamamuro, T., Matsusue, Y., Uchida, A., Shimada, K., Shimozaki, E. & Kitaoka, K. Bioabsorbable osteosynthetic implants of ultra high strength poly-L-lactide. Int. Orthop. 18, 332–340 (1994).
Langer, R. Drug delivery and targeting. Nature 392, 5–10 (1998).
Zhu, K.J., Xiangzhou, L. & Shilin, Y. Preparation, characterization, and properties of polylactide (PLA)-poly(ethylene glycol) (PEG) copolymers: a potential drug carrier. J. Appl. Polym. Sci. 39, 1–9 (1990).
Engelberg, I. & Kohn, J. Physico-mechanical properties of degradable polymers used in medical applications: a comparative study. Biomaterials 12, 292–304 (1991).
Prego, G., Cella, G.D. & Bastiolo, C. Effect of molecular weight and crystallinity on poly(lactic acid) mechanical properties. J. Appl. Polym. Sci. 59, 37–43 (1996).
Leenslag, J.W. & Pennings, A.J. Synthesis of high-molecular-weight poly(L-lactide) initiated with tin 2-ethylhezanoate. Macromol. Chem. 188, 1809–1814 (1987).
Mehta, R., Kumar, V., Bhunia, H. & Upadhyay, S.N. Synthesis of poly(lactic acid): a review. J. Macromol. Sci. C 45, 325–349 (2005).
Lu, L. & Mikos, A.G. Poly(glycolic acid). in Polymer Data Handbook (eds. Mark, J.E.) 566–569 (Oxford University Press, New York, 1999).
Lu, L. & Mikos, A.G. Poly(lactic acid). in Polymer Data Handbook (ed. Mark, J.E.) 627–633 (Oxford University Press, New York, 1999).
Nijenhuis, A.J., Colstee, E., Grijpma, D.W. & Pennings, A.J. High molecular weight poly(L-lactide) and poly(ethylene oxide) blends: thermal characterization and physical properties. Polymer 37, 5849–5857 (1996).
Carothers, W.H., Dorough, G.L. & van Natta, F.J. Studies of polymerization and ring formation. The reversible polymerization of six-membered cyclic esters. J. Am. Chem. Soc. 54, 761–772 (1932).
Sorensen, W.R. & Campbell, T.W. Preparative Methods of Polymer Chemistry 2nd edn. (Interscience Publishers Inc., New York, 1963).
Dechy-Cabaret, O., Martin-Vaca, B. & Bourissou, D. Controlled ring-opening polymerization of lactide and glycolide. Chem. Rev. 104, 6147–6176 (2004).
Krischeldorf, H.R., Kreiser-Saunders, I. & Stricker, A. Polylactones 48. SnOct2-initiated polymerizations of lactide: a mechanistic study. Macromolecules 33, 702–709 (2000).
Korhonen, H., Helminen, A. & Seppala, J.V. Synthesis of polylactides in the presence of co-initiators with different numbers of hydroxyl groups. Polymer 42, 7541–7549 (2001).
Hyon, S.-H., Jamshidi, K. & Ikada, Y. Synthesis of polylactides with different molecular weights. Biomaterials 18, 1503–1508 (1997).
Kricheldorf, H.R. & Serra, A. Polylactones. 6. Influence of various metal salts on the optical purity of poly(L-lactide). Polym. Bull. 14, 497–502 (2985).
Kricheldorf, H.R., Berl, M. & Scharnagl, N. Poly(lactones). 9. Polymerization mechanism of metal alkoxide initiated polymerizations of lactide and various lactones. Macromolecules 21, 286–293 (1988).
Biela, T., Kowalski, A., Libiszowski, J., Duda, A. & Penczek, S. Progress inpolymerzation of cyclic esters: mechanisms and synthetic applications. Macromol. Symp. 240, 47–55 (2006).
Acknowledgements
This work was supported by a Grant-in-Aid for General Scientific Research and by a Grant-in-Aid for the 21st Century COE Program 'KEIO LCC' from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. This work was also supported by a US National Institutes of Health grant to A.G.M. (R01 DE15164) as well as by the US National Science Foundation through a CAREER Award to J.P.F. (no. 0448684) and the Arthritis Foundation through an Arthritis Investigator Award to J.P.F.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaihara, S., Matsumura, S., Mikos, A. et al. Synthesis of poly(L-lactide) and polyglycolide by ring-opening polymerization. Nat Protoc 2, 2767–2771 (2007). https://doi.org/10.1038/nprot.2007.391
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.391
This article is cited by
-
The role of tri-n-butyltin(IV) n-butoxide/aluminum(III) tri-s-butoxide mixed initiators in the non-isothermal ring-opening polymerization of ε-caprolactone: from small-scale to larger-scale polymerization
Polymer Bulletin (2024)
-
Synthesis and characterization of lactide from Bacillus amyloliquefaciens brewed lactic acid utilizing cheap agricultural sources
3 Biotech (2024)
-
Ring-opening polymerization and plasticization of poly(L-lactic)acid by adding of glycerol-dioleate
Journal of Thermal Analysis and Calorimetry (2022)
-
Well-defined high molecular weight polyglycolide-b-poly(L-)lactide-b-polyglycolide triblock copolymers: synthesis, characterization and microstructural analysis
Journal of Polymer Research (2020)
-
Oligo(Lactic Acid)8-Rapamycin Prodrug-Loaded Poly(Ethylene Glycol)-block-Poly(Lactic Acid) Micelles for Injection
Pharmaceutical Research (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.