Abstract
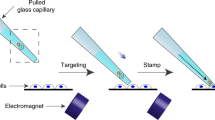
This protocol details how to design and conduct experiments to deliver nucleic acids to adherent and suspension cell cultures in vitro by magnetic force–assisted transfection using self-assembled complexes of nucleic acids and cationic lipids or polymers (nonviral gene vectors), which are associated with magnetic (nano) particles. These magnetic complexes are sedimented onto the surface of the cells to be transfected within minutes by the application of a magnetic gradient field. As the diffusion barrier to nucleic acid delivery is overcome, the full vector dose is targeted to the cell surface and transfection is synchronized. In this manner, the transfection process is accelerated and transfection efficiencies can be improved up to several 1,000-fold compared with transfections carried out with nonmagnetic gene vectors. This protocol describes how to accomplish the following stages: synthesis of magnetic nanoparticles for magnetofection; testing the association of DNA with the magnetic components of the transfection complex; preparation of magnetic lipoplexes and polyplexes; magnetofection; and data processing. The synthesis and characterization of magnetic nanoparticles can be accomplished within 3–5 d. Cell culture and transfection is then estimated to take 3 d. Transfected gene expression analysis, cell viability assays and calibration will probably take a few hours. This protocol can be used for cells that are difficult to transfect, such as primary cells, and may also be applied to viral nucleic acid delivery. With only minor alterations, this protocol can also be useful for magnetic cell labeling for cell tracking studies and, as it is, will be useful for screening vector compositions and novel magnetic nanoparticle preparations for optimized transfection efficiency in any cell type.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Plank, C., Scherer, F. & Rudolph, C. DNA Pharmaceuticals (ed. Schleef, M.) 55–116 (Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2005).
Felgner, P.L. et al. Nomenclature for synthetic gene delivery systems. Hum. Gene Ther. 8, 511–512 (1997).
Plank, C., Anton, M., Rudolph, C., Rosenecker, J. & Krotz, F. Enhancing and targeting nucleic acid delivery by magnetic force. Expert Opin. Biol. Ther. 3, 745–758 (2003).
Hughes, C., Galea-Lauri, J., Farzaneh, F. & Darling, D. Streptavidin paramagnetic particles provide a choice of three affinity-based capture and magnetic concentration strategies for retroviral vectors. Mol. Ther. 3, 623–630 (2001).
Mah, C. et al. Improved method of recombinant AAV2 delivery for systemic targeted gene therapy. Mol. Ther. 6, 106–112 (2002).
Pandori, M.W., Hobson, D.A. & Sano, T. Adenovirus-microbead conjugates possess enhanced infectivity: a new strategy to localized gene delivery. Virology 299, 204–212 (2002).
Hirao, K. et al. Targeted gene delivery to human osteosarcoma cells with magnetic cationic liposomes under a magnetic field. Int. J. Oncol. 22, 1065–1071 (2003).
Satoh, K. et al. Virus concentration using polyethyleneimine-conjugated magnetic beads for improving the sensitivity of nucleic acid amplification tests. J. Virol. Methods 114, 11–19 (2003).
Xiang, J.J. et al. IONP-PLL: a novel non-viral vector for efficient gene delivery. J. Gene Med. 5, 803–817 (2003).
Campos, S.K., Parrott, M.B. & Barry, M.A. Avidin-based targeting and purification of a protein IX-modified, metabolically biotinylated adenoviral vector. Mol. Ther. 9, 942–954 (2004).
Raty, J.K. et al. Enhanced gene delivery by avidin-displaying baculovirus. Mol. Ther. 9, 282–291 (2004).
Chan, L. et al. Conjugation of lentivirus to paramagnetic particles via nonviral proteins allows efficient concentration and infection of primary acute myeloid leukemia cells. J. Virol. 79, 13190–13194 (2005).
Haim, H., Steiner, I. & Panet, A. Synchronized infection of cell cultures by magnetically controlled virus. J. Virol. 79, 622–625 (2005).
Kadota, S., Kanayama, T., Miyajima, N., Takeuchi, K. & Nagata, K. Enhancing of measles virus infection by magnetofection. J. Virol. Methods 128, 61–66 (2005).
Morishita, N. et al. Magnetic nanoparticles with surface modification enhanced gene delivery of HVJ-E vector. Biochem. Biophys. Res. Commun. 334, 1121–1126 (2005).
Isalan, M., Santori, M.I., Gonzalez, C. & Serrano, L. Localized transfection on arrays of magnetic beads coated with PCR products. Nat. Methods 2, 113–118 (2005).
Hafeli, U.O. Magnetically modulated therapeutic systems. Int. J. Pharm. 277, 19–24 (2004).
Widder, K.J., Senyel, A.E. & Scarpelli, G.D. Magnetic microspheres: a model system of site specific drug delivery in vivo. Proc. Soc. Exp. Biol. Med. 158, 141–146 (1978).
Mah, C. et al. Microsphere-mediated delivery of recombinant AAV vectors in vitro and in vivo. Mol. Ther. 1, S239 (2000).
Plank, C., Scherer, F., Schillinger, U. & Anton, M. Magnetofection: enhancement and localization of gene delivery with magnetic particles under the influence of a magnetic field. J. Gene. Med. 2, 24 (2000).
Scherer, F. et al. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene. Ther. 9, 102–109 (2002).
Nesbeth, D. et al. Metabolic biotinylation of lentiviral pseudotypes for scalable paramagnetic microparticle-dependent manipulation. Mol. Ther. 13, 814–822 (2006).
Huth, S. et al. Insights into the mechanism of magnetofection using PEI-based magnetofectins for gene transfer. J. Gene Med. 6, 923–936 (2004).
Weissleder, R. et al. Superparamagnetic iron oxide: pharmacokinetics and toxicity. AJR Am. J. Roentgenol. 152, 167–173 (1989).
Schillinger, U. et al. Advances in magnetofection—magnetically guided nucleic acid delivery. J. Magnetism Magnetic Mater. 293, 501–508 (2005).
Plank, C. et al. The magnetofection method: using magnetic force to enhance gene delivery. Biol. Chem. 384, 737–747 (2003).
Dobson, J. Gene therapy progress and prospects: magnetic nanoparticle-based gene delivery. Gene Ther. 13, 283–287 (2006).
Smith, C. Sharpening the tools of RNA interference. Nat. Methods 3, 475–486 (2006).
Krotz, F. et al. Magnetofection—a highly efficient tool for antisense oligonucleotide delivery in vitro and in vivo. Mol. Ther. 7, 700–710 (2003).
Boussif, O. et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. USA 92, 7297–7301 (1995).
Riess, JG. Fluorous micro-and nanophases with a biomedical perspective. Tetrahedron Fluorous Chem. 58, 4113–4131 (2002).
Mykhaylyk, O. et al. Magnetic nanoparticle formulations for DNA and siRNA delivery. J. Magnetism Magnetic Mater. 311, 275–281 (2007).
Mikhaylova, M. et al. Superparamagnetism of magnetite nanoparticles: dependence on surface modification. Langmuir 20, 2472–2477 (2004).
Prozorov, R., Yeshurun, Y., Prozorov, T. & Gedanken, A. Magnetic irreversibility and relaxation in assembly of ferromagnetic nanoparticles. Phys. Rev. B 59, 6956LP–6965 (1999).
Kowalski, J.B. & Tallentire, A. Substantiation of 25 kGy as a sterilization dose: a rational approach to establishing verification dose. Radiat. Phys. Chem. 54, 55–64 (1999).
Azzam, T. & Domb, A.J. Current developments in gene transfection agents. Curr. Drug Deliv. 1, 165–193 (2004).
Bonetta, L. The inside scoop—evaluating gene delivery methods. Nat. Methods 2, 875–883 (2005).
Moghimi, S.M. et al. A two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy. Mol. Ther. 11, 990–995 (2005).
Wightman, L. et al. Different behavior of branched and linear polyethylenimine for gene delivery in vitro and in vivo. J. Gene Med. 3, 362–372 (2001).
Lungwitz, U., Breunig, M., Blunk, T. & Gopferich, A. Polyethylenimine-based non-viral gene delivery systems. Eur. J. Pharm. Biopharm. 60, 247–266 (2005).
Boeckle, S. et al. Purification of polyethylenimine polyplexes highlights the role of free polycations in gene transfer. J. Gene Med. 6, 1102–1111 (2004).
Mueller, H., Kassack, M.U. & Wiese, M. Comparison of the usefulness of the MTT, ATP, and calcein assays to predict the potency of cytotoxic agents in various human cancer cell lines. J. Biomol. Screen. 9, 506–515 (2004).
Berridge, M.V., Herst, P.M. & Tan, A.S. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnol. Annu. Rev. 11, 127–152 (2005).
Berridge, M.V., Tan, A.S. & Hilton, C.J. Cyclic adenosine monophosphate promotes cell survival and retards apoptosis in a factor-dependent bone marrow-derived cell line. Exp. Hematol. 21, 269–276 (1993).
Terebesi, J., Kwok, K.Y. & Rice, K.G. Iodinated plasmid DNA as a tool for studying gene delivery. Anal. Biochem. 263, 120–123 (1998).
Krieg, A.M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20, 709–760 (2002).
Acknowledgements
The authors would like to thank E. Hammerschmid for the help with the flow cytometry analysis, and Dr. Joachim Aigner for the help with microscopy studies and valuable discussions. This work was supported by the European Union through the FP6-LIFESCIHEALTH Project “Improved precision of nucleic acid based therapy of cystic fibrosis” under contract no. 005213 as well as by the German Ministry of Education and Research, Nanobiotechnology grants 13N8186 and 13N8538. Financial support of the German Excellence Initiative via the “Nanosystems Initiative Munich (NIM)” is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Christian Plank is a co-founder of OZ Biosciences, Marseille, France. OZ Biosciences manufactures and sells magnetofection reagents.
Rights and permissions
About this article
Cite this article
Mykhaylyk, O., Antequera, Y., Vlaskou, D. et al. Generation of magnetic nonviral gene transfer agents and magnetofection in vitro. Nat Protoc 2, 2391–2411 (2007). https://doi.org/10.1038/nprot.2007.352
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2007.352
This article is cited by
-
Biosynthesis of ternary NiCoFe2O4 nanoflowers: investigating their 3D structure and potential use in gene delivery
Journal of Biological Engineering (2023)
-
Heterologous Expression of Codon-Optimized Azurin Transferred by Magnetofection Method in MCF-10A Cells
Molecular Biotechnology (2023)
-
Progressive lysosomal membrane permeabilization induced by iron oxide nanoparticles drives hepatic cell autophagy and apoptosis
Nano Convergence (2020)
-
DNA–Iron Oxide Nanoparticles Conjugates: Functional Magnetic Nanoplatforms in Biomedical Applications
Topics in Current Chemistry (2020)
-
Bacterial magnetic particles-polyethylenimine vectors deliver target genes into multiple cell types with a high efficiency and low toxicity
Applied Microbiology and Biotechnology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.