Abstract
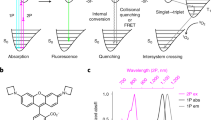
The ability to directly image and quantify drug-target engagement and drug distribution with subcellular resolution in live cells and whole organisms is a prerequisite to establishing accurate models of the kinetics and dynamics of drug action. Such methods would thus have far-reaching applications in drug development and molecular pharmacology. We recently presented one such technique based on fluorescence anisotropy, a spectroscopic method based on polarization light analysis and capable of measuring the binding interaction between molecules. Our technique allows the direct characterization of target engagement of fluorescently labeled drugs, using fluorophores with a fluorescence lifetime larger than the rotational correlation of the bound complex. Here we describe an optimized protocol for simultaneous dual-channel two-photon fluorescence anisotropy microscopy acquisition to perform drug-target measurements. We also provide the necessary software to implement stream processing to visualize images and to calculate quantitative parameters. The assembly and characterization part of the protocol can be implemented in 1 d. Sample preparation, characterization and imaging of drug binding can be completed in 2 d. Although currently adapted to an Olympus FV1000MPE microscope, the protocol can be extended to other commercial or custom-built microscopes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout















Similar content being viewed by others
References
Vinegoni, C. et al. Advances in measuring single-cell pharmacokinetics and pharmacology in vivo. Drug Discov. Today 20, 1087–1092 (2015).
Miller, M.A. et al. Predicting therapeutic nanomedicine efficacy using a companion magnetic resonance imaging nanoparticle. Sci. Transl. Med. 7, 314 (2015).
Laughney, A.M. et al. Single-cell pharmacokinetic imaging reveals a therapeutic strategy to overcome drug resistance to the microtubule inhibitor eribulin. Sci. Transl. Med. 6, 261 (2014).
Dubach, J.M. et al. In vivo imaging of specific drug-target binding at subcellular resolution. Nat. Commun. 5, 3946 (2014).
Molina, D.M. et al. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 341, 84–87 (2013).
Savitski, M.M. et al. Tracking cancer drugs in living cells by thermal profiling of the proteome. Science 346, 1255784 (2014).
Grimwood, S. & Hartig, P.R. Target site occupancy: emerging generalizations from clinical and preclinical studies. Pharmacol. Ther. 122, 281–301 (2009).
Matthews, P.M., Rabiner, E.A., Passchier, J. & Gunn, R.N. Positron emission tomography molecular imaging for drug development. Br. J. Clin. Pharmacol. 73, 175–186 (2012).
Liu, X.H. et al. Molecular imaging of drug transit through the blood–brain barrier with MALDI mass spectrometry imaging. Sci. Rep. 3, 2859 (2013).
Miller, M.A. & Weissleder, R. Imaging the pharmacology of nanomaterials by intravital microscopy: toward understanding their biological behavior. Adv. Drug Deliv. Rev. http://dx.doi.org/10.1016/j.addr.2016.05.023 (2016).
Svoboda, K. & Yasuda, R. Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron 50, 823–839 (2006).
Gough, A.H. & Taylor, D.L. Fluorescence anisotropy imaging microscopy maps calmodulin-binding during cellular contraction and locomotion. J. Cell Biol. 121, 1095–1107 (1993).
Lakowicz, J.R. Principles of Fluorescence Spectroscopy (Plenum Press, 1983).
Lakowicz, J.R. Principles of Fluorescence Spectroscopy 2nd edn. (Kluwer Academic/Plenum Publishers, 1999).
Dix, J.A. & Verkman, A.S. Mapping of fluorescence anisotropy in living cells by ratio imaging. Biophys. J. 57, 231–240 (1990).
Swaminathan, R., Hoang, C.P. & Verkman, A.S. Photobleaching recovery and anisotropy decay of green fluorescent protein GFP-S65T in solution and cells: cytoplasmic viscosity probed by green fluorescent protein translational and rotational diffusion. Biophys. J. 72, 1900–1907 (1997).
Foster, T.H., Pearson, B.D., Mitra, S. & Bigelow, C.E. Fluorescence anisotropy imaging reveals localization of meso-tetrahydroxyphenyl chlorin in the nuclear envelope. Photochem. Photobiol. 81, 1544–1547 (2005).
Cao, Z., Huang, C.C. & Tan, W. Nuclease resistance of telomere-like oligonucleotides monitored in live cells by fluorescence anisotropy imaging. Anal. Chem. 78, 1478–1484 (2006).
Bigelow, C.E., Vishwasrao, H.D., Frelinger, J.G. & Foster, T.H. Imaging enzyme activity with polarization-sensitive confocal fluorescence microscopy. J. Microsc. 215, 24–33 (2004).
Li, W., Wang, Y., Shao, H., He, Y. & Ma, H. Probing rotation dynamics of biomolecules using polarization based fluorescence microscopy. Microsc. Res. Tech. 70, 390–395 (2007).
Bigelow, C.E., Conover, D.L. & Foster, T.H. Confocal fluorescence spectroscopy and anisotropy imaging system. Opt. Lett. 28, 695–697 (2003).
Chan, F.T.S., Kaminski, C.F. & Kaminski-Schierle, G.S. HomoFRET fluorescence anisotropy imaging as a tool to study molecular self-assembly in live cells. ChemPhysChem. 12, 500–509 (2011).
Hedde, P.N., Ranjit, S. & Gratton, E. 3D fluorescence anisotropy imaging using selective plane illumination microscopy. Opt. Express 23, 22308–22317 (2015).
Yengo, C.M. & Berger, C.L. Fluorescence anisotropy and resonance energy transfer: powerful tools for measuring real time protein dynamics in a physiological environment. Curr. Opin. Pharmacol. 10, 731–737 (2010).
Gradinaru, C.C., Marushchak, D.O., Samim, M. & Krull, U.J. Fluorescence anisotropy: from single molecules to live cells. Analyst 135, 452–459 (2010).
Suhling, K., Levitt, J. & Chung, P.H. Time-resolved fluorescence anisotropy imaging. In Fluorescence Spectroscopy and Microscopy (eds. Engelborghs, Y. & Visser, J.W.G.) (Springer Science, 2014).
Jameson, D.M. & Ross, J.A. Fluorescence polarization/anisotropy in diagnostic and imaging. Chem. Rev. 110, 2685–2708 (2010).
Matthews, D.R. et al. Time-lapse FRET microscopy using fluorescence anisotropy. J. Microsc. 237, 51–62 (2010).
Turetsky, A., Kim, E., Kohler, R.H., Miller, M.A. & Weissleder, R. Single cell imaging of Bruton's tyrosine kinase using an irreversible inhibitor. Sci. Rep. 4, 4782 (2014).
Reiner, T. et al. Imaging therapeutic PARP inhibition in vivo through bioorthogonally developed companion imaging agents. Neoplasia 14, 169–177 (2012).
Thurber, G.M. et al. Single-cell and subcellular pharmacokinetic imaging allows insight into drug action in vivo. Nat. Commun. 4, 1504 (2013).
Hottiger, M.O. Nuclear ADP-ribosylation and its role in chromatin plasticity, cell differentiation, and epigenetics. Annu. Rev. Biochem. 84, 227–263 (2015).
Barkauskaite, E., Jankevicius, G. & Ahel, I. Structures and mechanisms of enzymes employed in the synthesis and degradation of PARP-dependent protein ADP-ribosylation. Mol. Cell 58, 935–946 (2015).
Caldecott, K.W. Protein ADP-ribosylation and the cellular response to DNA strand breaks. DNA Repair 19, 108–113 (2014).
Scott, C.L., Swisher, E.M. & Kaufmann, S.H. Poly (ADP-ribose) polymerase inhibitors: recent advances and future development. J. Clin. Oncol. 33, 1397–1406 (2015).
Curtin, N.J. & Szabo, C. Therapeutic applications of PARP inhibitors: anticancer therapy and beyond. Mol. Aspects Med. 34, 1217–1256 (2013).
O'Connor, M.J. Targeting the DNA damage response in cancer. Mol. Cell 60, 547–560 (2015).
Shen, Y., Aoyagi-Scharber, M. & Wang, B. Trapping poly (ADP-ribose) polymerase. J. Pharmacol. Exp. Ther. 353, 446–457 (2015).
Rouleau, M., Patel, A., Hendzel, M.J., Kaufmann, S.H. & Poirier, G.G. PARP inhibition: PARP1 and beyond. Nat. Rev. Cancer 10, 293–301 (2010).
Mateo, J. et al. DNA-repair defects and olaparib in metastatic prostate cancer. N. Engl. J. Med. 373, 1697–1708 (2015).
Ossovskaya, V., Koo, I.C., Kaldjian, E.P., Alvares, C. & Sherman, B.M. Upregulation of poly (ADP-ribose) polymerase-1 (PARP1) in triple-negative breast cancer and other primary human tumor types. Genes Cancer 1, 812–821 (2010).
Bièche, I., Murcia, G.D. & Lidereau, R. Poly (ADP-ribose) polymerase gene expression status and genomic instability in human breast cancer. Clin. Cancer Res. 2, 1163–1167 (1996).
Rojo, F. et al. Nuclear PARP-1 protein overexpression is associated with poor overall survival in early breast cancer. Ann. Oncol. 23, 1156–1164 (2012).
Alanazi, M. et al. Association between PARP-1 V762A polymorphism and breast cancer susceptibility in Saudi population. PLoS One 8, e855411 (2013).
Galia, A. et al. PARP-1 protein expression in glioblastoma multiforme. Eur. J. Histochem. 56, e9 (2012).
Barton, V.N. et al. PARP1 expression in pediatric central nervous system tumors. Pediatr. Blood Cancer 53, 1227–1230 (2009).
Staibano, S. et al. Poly (adenosine diphosphate-ribose) polymerase 1 expression in malignant melanomas from photoexposed areas of the head and neck region. Hum. Pathol. 36, 724–731 (2005).
Reiner, T., Earley, S., Turetsky, A. & Weissleder, R. Bioorthogonal small-molecule ligands for PARP1 imaging in living cells. Chembiochem 11, 2374–2377 (2010).
Thurber, G.M. et al. Effect of small-molecule modification on single-cell pharmacokinetics of PARP inhibitors. Mol. Cancer Ther. 13, 986–995 (2014).
Irwin, C.P. et al. PARPi-FL – a fluorescent PARP1 inhibitor for glioblastoma imaging. Neoplasia 16, 432–440 (2014).
Kossatz, S. et al. Detection and delineation of oral cancer with a PARP1 targeted optical imaging agent. Sci. Rep. 6, 21371 (2016).
Yan, Y. & Marriot, G. Fluorescence resonance energy transfer imaging microscopy and fluorescence polarization imaging microscopy. Methods Enzymol. 360, 561–580 (2003).
Koshioka, M., Sasaki, K. & Masuhara, H. Time-dependent fluorescence depolarization analysis in three-dimensional microspectroscopy. Appl. Spectrosc. 49, 224–228 (1995).
Ha, T., Laurence, T.A., Chemla, D.S. & Weiss, S. Polarization spectroscopy of single fluorescent molecules. J. Phys. Chem. B 103, 6839–6850 (1999).
Becker, W. Advanced Time-Correlated Single Photon Counting Techniques. (eds. Castleman, A.W., Toennis, J.P., & Zinth, W.) (Springer Series in Chemical Physics, 2005).
Jameson, D.M., Weber, G., Spencer, R.D. & Mitchell, G. Fluorescence polarization: measurements with a photon counting photometer. Rev. Sci. Instrum. 49, 510–514 (1978).
Benninger, R.K.P., Ashby, W.J., Ring, E.A. & Piston, D.W. A single-photon counting detector for increased sensitivity in two-photon laser scanning microscopy. Opt. Lett. 33, 2895–2897 (2008).
Moon, S. & Kim, D.Y. Analog single-photon counter for high-speed scanning microscopy. Opt. Express 16, 13990–14003 (2008).
Hoover, E.E. & Squier, J.A. Advances in multiphoton microscopy technology. Nat. Photonics 7, 93–101 (2013).
Driscoll, J.D. et al. Photon counting, censor corrections, and lifetime imaging for improved detection in two-photon microscopy. J. Neurophysiol. 105, 3106–3113 (2011).
Buehler, C., Kim, K.H., Greuter, U., Schlumpf, N. & So, P.T.C. Single-photon counting multicolor multiphoton fluorescence microscope. J. Fluoresc. 15, 41–51 (2005).
Dix, J.A. & Verkman, A.S. Mapping of fluorescence anisotropy in living cells by ratio imaging: application to cytoplasmic viscosity. Biophys. J. 57, 231–240 (1990).
Thompson, R.B., Gryczynski, I. & Malicka, J. Fluorescence polarization standards for high-throughput screening and imaging. BioTechniques 32, 42 (2002).
van Kempen, G.M.P. & van Vliet, L.J. Mean and variance of ratio estimators used in fluorescence ratio imaging. Cytometry 39, 300–305 (2000).
Lidke, K.A., Rieger, B., Lidke, D.S. & Jovin, T.M. The role of photon statistics in fluorescence anisotropy imaging. IEEE Trans. Image Process. 14, 1237–1245 (2005).
Siegel, J. et al. Studying biological tissue with fluorescence lifetime imaging: microscopy, endoscopy, and complex decay profiles. Appl. Opt. 42, 2995–3004 (2003).
Suhling, K. et al. Time-resolved fluorescence anisotropy imaging (TR-FAIM) applied to live cells. Opt. Lett. 29, 584–586 (2004).
Xu, C. & Webb, W.W. Measurement of two-photon excitation cross sections of molecular fluorophores with data from 690 to 1050 nm. J. Opt. Soc. Am. B 13, 481–491 (1996).
Weber, G. Dependence of polarization of the fluorescence on the concentration. Trans. Faraday Soc. 50, 552–555 (1954).
Yang, K.S., Budin, G., Reiner, T., Vinegoni, C. & Weissleder, R. Bioorthogonal imaging of aurora kinase A in live cells. Angew. Chem. Int. Ed. Engl. 51, 6598–6603 (2012).
Kim, E. et al. Optimized near-IR fluorescent agents for in vivo imaging of Btk expression. Bioconjug. Chem. 26, 1513–1518 (2015).
Fu, W. et al. Epigenetic modulation of type-1 diabetes via a dual effect on pancreatic macrophages and cells. eLife 3, e04631 (2014).
Mikula, H., Stapleton, S., Kohler, R.H., Vinegoni, C. & Weissleder, R. Design and development of fluorescent vemurafenib analogs for in vivo imaging. Theranostics 7, 1257–1265 (2017).
Kim, E., Yang, K. & Weissleder, R. Bioorthogonal small molecule imaging agents allow single-cell imaging of MET. PLoS One 8, e81275 (2013).
Miller, M.A. et al. Tumour-associated macrophages act as a slow-release reservoir of nanotherapeutic Pt(IV) pro-drug. Nat. Commun. 6, 8692 (2015).
Earley, S. et al. In vivo imaging of drug-induced mitochondrial outer membrane permeabilization at single cell resolution. Cancer Res. 72, 2949–2956 (2012).
Yang, K.S., Budin, G., Tassa, C., Kister, O. & Weissleder, R. Bioorthogonal approach to identify unsuspected drug targets in live cells. Angew. Chem. Int. Ed. Engl. 52, 10593–10597 (2013).
Yang, K.S., Kohler, R.H., Landon, M., Giedt, R. & Weissleder, R. Single cell resolution in vivo imaging of DNA damage following PARP inhibition. Sci. Rep. 5, 10129 (2015).
Miller, M.A., Askevold, B., Yang, K.S., Kohler, R.H. & Weissleder, R. Platinum compounds for high-resolution in vivo cancer imaging. Chem. Med. Chem. 9, 1131–1135 (2014).
Song, Y., Suntharalingam, K., Yeung, J.S., Royzen, M. & Lippard, S.J. Synthesis and characterization of Pt(IV) fluorescein conjugates to investigate Pt(IV) intracellular transformations. Bioconjug. Chem. 24, 1733–1740 (2013).
Orth, J.D. et al. Analysis of mitosis and antimitotic drug responses in tumors by in vivo microscopy and single-cell pharmacodynamics. Cancer Res. 71, 4608–4616 (2011).
Chittajallu, D.R. et al. In vivo cell-cycle profiling in xenograft tumors by quantitative intravital microscopy. Nat. Methods 12, 577–585 (2015).
Meimetis, L.G. et al. Fluorescent vinblastine probes for live cell imaging. Chem. Commun. 52, 9953–9956 (2016).
Acknowledgements
This project was funded in part by National Institutes of Health (NIH) grants RO1-EB010011, RO1-HL122208 and P50GM107618. Investigators at Memorial Sloan Kettering Cancer Center were funded in part by NIH grant P30 CA008748 and investigators in Europe were funded by the EC Seventh Framework Programme under grant agreement no. 622182.
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing of the paper. C.V., R.M., P.F.F. and R.W., along with others, primarily developed the technique. S. Shah and I.G. conducted one-photon experiments. C.V. and P.F.F. wrote the software. S.L. designed and machined the systems components. A.E.N., C.B., R.M. and T.R. performed the synthesis. S. Stapleton prepared cell cultures.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 PARPi-FL.
Chemical structure (a) and analytical HPLC chromatogram (b) of PARPi-FL.
Supplementary Figure 2 Image Acquisition Control window of the Olympus FluoView program.
The interface allows to control the PMT voltage settings, to initiate acquisitions and image averaging. Image of FLUOVIEW program adapted with permission from Olympus.
Supplementary Figure 3 Acquisition Setting window of the Olympus FluoView program.
The interface allows to set image acquisition dwell time, size, zoom factor, excitation wavelength selection and laser power. Image of FLUOVIEW program adapted with permission from Olympus.
Supplementary Figure 4 Acquired Image window of the Olympus FluoView program.
The two images (red boxes 1,2) show simultaneous acquisition of fluorescence emission at two orthogonal state of polarization as detected at PMT3 and PMT4, for a solution of fluorescein diluted in a mixture of water-glycerol (w/w) at 95%. Image of FLUOVIEW program adapted with permission from Olympus.
Supplementary Figure 5 Alignment polarization control unit.
Laser intensity as measured at the back-aperture of the objective, as a function of the half-waveplate angle. Light is measured after passing through a near infrared linear polarizer parallel to the microscope's Y axis (Fig. 4a).
Supplementary Figure 6 LightPath & Dyes window of the Olympus FluoView program.
The interface allows to select the dichroic, imaging path, and the imaging PMTs for simultaneous dual channel detection. Image of FLUOVIEW program adapted with permission from Olympus.
Supplementary Figure 7 GUI interface of the BackgroundEstimation.exe.
GUI interface of the BackgroundEstimation program, necessary to calculate the dark noise background correction.
Supplementary Figure 8 Closed bath imaging chamber.
(a-c) Different views of the closed bath imaging chamber for time measurements of drug target engagement. 1, inflow tubing connected to the perfusion manifold; 2, cell-seeded coverslip; 3, tubing connected to the vacuum outlet; 4, suction reservoir. The 2x dry objective, is used to find cell-seeded areas on the imaging coverslip.
Supplementary Figure 9 GUI interface of the OffLine.exe.
GUI interface of the OffLine program, for image processing, calculation and visualization of fluorescence anisotropy images for data previously acquired.
Supplementary information
Supplementary Text and Figures
Supplementary Methods and Supplementary Figures 1–9. (PDF 1979 kb)
Supplementary Data 1
A .zip file containing the STL file for the assembly of the polarization control unit. (ZIP 8201 kb)
Supplementary Data 2
A .zip file containing the STL files for the 3D printing and drawing of the polarization filter cube. (ZIP 331 kb)
Supplementary Data 3
A .zip file containing a longitudinal data set acquired in a perfusion chamber during the drug loading and washing phase, calibration measurements for high and low values of anisotropy, and data for cells at different time points during the loading and washing time. (ZIP 13205 kb)
Supplementary Software
A .zip file containing the software for the OnLine and OffLine elaboration and visualization of the fluorescence anisotropy images, and for calculating the background noise subtraction and average anisotropy: OnLine.exe, OffLine.exe, BackgroundEstimation.exe, AnisotropyCalculation.exe. (ZIP 23538 kb)
Rights and permissions
About this article
Cite this article
Vinegoni, C., Fumene Feruglio, P., Brand, C. et al. Measurement of drug-target engagement in live cells by two-photon fluorescence anisotropy imaging. Nat Protoc 12, 1472–1497 (2017). https://doi.org/10.1038/nprot.2017.043
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.043
This article is cited by
-
Bright and stable luminescent probes for target engagement profiling in live cells
Nature Chemical Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.