Abstract
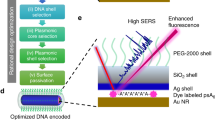
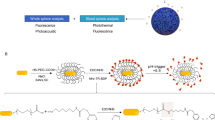
The unique spectral signatures and biologically inert compositions of surface-enhanced resonance Raman scattering (SERRS) nanoparticles make them promising contrast agents for in vivo cancer imaging. Our SERRS nanoparticles consist of a 60-nm gold nanoparticle core that is encapsulated in a 15-nm-thick silica shell wherein the resonant Raman reporter is embedded. Subtle aspects of their preparation can shift their limit of detection by orders of magnitude. In this protocol, we present the optimized, step-by-step procedure for generating reproducible SERRS nanoparticles with femtomolar (10−15 M) limits of detection. We provide ways of characterizing the optical properties of SERRS nanoparticles using UV/VIS and Raman spectroscopy, and their physicochemical properties using transmission electron microscopy and nanoparticle tracking analysis. We introduce several applications of these nanoprobes for biomedical research, with a focus on intraoperative cancer imaging via Raman imaging. A detailed account is provided for successful i.v. administration of SERRS nanoparticles such that delineation of cancerous lesions can be achieved in vivo and ex vivo on resected tissues without the need for specific biomarker targeting. This straightforward, yet comprehensive, protocol—from initial de novo gold nanoparticle synthesis to SERRS nanoparticle contrast-enhanced preclinical Raman imaging in animal models—takes ∼96 h.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Andreou, C., Kishore, S.A. & Kircher, M.F. Surface-enhanced Raman spectroscopy: a new modality for cancer maging. J. Nucl. Med. 56, 1295–1299 (2015).
Pence, I. & Mahadevan-Jansen, A. Clinical instrumentation and applications of Raman spectroscopy. Chem. Soc. Rev. 45, 1958–1979 (2016).
Long, D.A. Raman Spectroscopy (McGraw-Hill, 1977).
Freudiger, C.W. et al. Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science 322, 1857–1861 (2008).
Saar, B.G. et al. Video-rate molecular imaging in vivo with stimulated Raman scattering. Science 330, 1368–1370 (2010).
Kircher, M.F. et al. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat. Med. 18, 829–834 (2012).
Wang, Y.Q., Yan, B. & Chen, L.X. SERS tags: novel optical nanoprobes for bioanalysis. Chem. Rev. 113, 1391–1428 (2013).
Hong, S.M. & Li, X. Optimal size of gold nanoparticles for surface-enhanced Raman spectroscopy under different conditions. J. Nanomater. http://dx.doi.org/10.1155/2013/790323 (2013).
Harmsen, S. et al. Rational design of a chalcogenopyrylium-based surface-enhanced resonance Raman scattering nanoprobe with attomolar sensitivity. Nat. Commun 6, 6570 (2015).
Maeda, H., Wu, J., Sawa, T., Matsumura, Y. & Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Release 65, 271–284 (2000).
Lombardi, J.R. & Birke, R.L. The theory of surface-enhanced Raman scattering. J. Chem. Phys. 136, 144704 (2012).
Lombardi, J.R. & Birke, R.L. A unified view of surface-enhanced Raman scattering. Acc. Chem. Res. 42, 734–742 (2009).
Harmsen, S. et al. Surface-enhanced resonance Raman scattering nanostars for high-precision cancer imaging. Sci. Transl. Med. 7, 271ra277 (2015).
Fales, A.M., Yuan, H. & Vo-Dinh, T. Silica-coated gold nanostars for combined surface-enhanced Raman scattering (SERS) detection and singlet-oxygen generation: a potential nanoplatform for theranostics. Langmuir 27, 12186–12190 (2011).
Mulvaney, S.P., Musick, M.D., Keating, C.D. & Natan, M.J. Glass-coated, analyte-tagged nanoparticles: a new tagging system based on detection with surface-enhanced Raman scattering. Langmuir 19, 4784–4790 (2003).
Qian, X. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat. Biotechnol 26, 83–90 (2008).
Spaliviero, M. et al. Detection of lymph node metastases with SERRS nanoparticles. Mol. Imaging Biol. 18, 677–685 (2016).
Garai, E. et al. High-sensitivity, real-time, ratiometric imaging of surface-enhanced Raman scattering nanoparticles with a clinically translatable Raman endoscope device. J. Biomed. Opt. 18, 096008 (2013).
Thakor, A.S. et al. The fate and toxicity of Raman-active silica-gold nanoparticles in mice. Sci. Transl. Med. 3, 79ra33 (2011).
Thakor, A.S. & Gambhir, S.S. Nanooncology: the future of cancer diagnosis and therapy. CA Cancer J. Clin. 63, 395–418 (2013).
Huang, R. et al. High precision imaging of microscopic spread of glioblastoma with a targeted ultrasensitive SERRS molecular imaging probe. Theranostics 6, 1075–1084 (2016).
Bodelon, G. et al. Au@pNIPAM SERRS tags for multiplex immunophenotyping cellular receptors and imaging tumor cells. Small 11, 4149–4157 (2015).
Wang, Y.W. et al. Rapid ratiometric biomarker detection with topically applied SERS nanoparticles. Technology (Singap. World Sci.) 2, 118–132 (2014).
Nima, Z.A. et al. Circulating tumor cell identification by functionalized silver-gold nanorods with multicolor, super-enhanced SERS and photothermal resonances. Sci. Rep. 4, 4752 (2014).
Shaffer, T.M. et al. Silica nanoparticles as substrates for chelator-freelabeling of oxophilic radioisotopes. Nano Lett. 15, 864–868 (2015).
Shaffer, T.M. et al. Stable radiolabeling of sulfur-functionalized silica nanoparticles with Copper-64. Nano Lett. 16, 5601–5604 (2016).
Mulder, W.J., Jaffer, F.A., Fayad, Z.A. & Nahrendorf, M. Imaging and nanomedicine in inflammatory atherosclerosis. Sci. Transl. Med. 6, 239sr231 (2014).
Suarez, S., Almutairi, A. & Christman, K.L. Micro- and nanoparticles for treating cardiovascular disease. Biomater. Sci. 3, 564–580 (2015).
Kircher, M.F. & Willmann, J.K. Molecular body imaging: MR imaging, CT, and US. Part II. Applications. Radiology 264, 349–368 (2012).
Kircher, M.F. & Willmann, J.K. Molecular body imaging: MR imaging, CT, and US. Part I. Principles. Radiology 263, 633–643 (2012).
de Boer, E. et al. Optical innovations in surgery. Br. J. Surg. 102, e56–72 (2015).
Andreou, C. et al. Imaging of liver tumors using surface-enhanced Raman scattering nanoparticles. ACS Nano 10, 5015–5026 (2016).
von Maltzahn, G. et al. SERS-coded gold nanorods as a multifunctional platform for densely multiplexed near-infrared imaging and photothermal heating. Adv. Mater. 21, 3175–3180 (2009).
Fernandez-Lopez, C. et al. Highly controlled silica coating of PEG-capped metal nanoparticles and preparation of SERS-encoded particles. Langmuir 25, 13894–13899 (2009).
Fales, A.M., Yuan, H. & Vo-Dinh, T. Development of hybrid silver-coated gold nanostars for nonaggregated surface-enhanced Raman scattering. J. Phys. Chem. C Nanomater. Interfaces 118, 3708–3715 (2014).
Khoury, C.G. & Vo-Dinh, T. Gold nanostars for surface-enhanced Raman scattering: synthesis, characterization and optimization. J. Phys. Chem. C 112, 18849–18859 (2008).
Mir-Simon, B., Reche-Perez, I., Guerrini, L., Pazos-Perez, N. & Alvarez-Puebla, R.A. Universal one-pot and scalable synthesis of SERS encoded nanoparticles. Chem. Mater. 27, 950–958 (2015).
Yuan, H. et al. Quantitative surface-enhanced resonant Raman scattering multiplexing of biocompatible gold nanostars for in vitro and ex vivo detection. Anal. Chem. 85, 208–212 (2013).
Turkevich, J., Stevenson, P.C. & Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 55 http://dx.doi.org/10.1039/df9511100055 (1951).
Wall, M.A., et al. Surfactant-free shape control of gold nanoparticles enabled by unified theoretical framework of nanocrystal synthesis. Adv. Mat http://dx.doi.org/10.1002/adma.201605622 (2017).
Stober, W., Fink, A. & Bohn, E. Controlled growth of monodisperse silica spheres in micron size range. J. Colloid Interface Sci. 26, 62–69 (1968).
Han, X., Goebl, J., Lu, Z. & Yin, Y. Role of salt in the spontaneous assembly of charged gold nanoparticles in ethanol. Langmuir 27, 5282–5289 (2011).
Bohndiek, S.E. et al. A small animal Raman instrument for rapid, wide-area, spectroscopic imaging. Proc. Natl. Acad. Sci. USA 110, 12408–12413 (2013).
Palonpon, A.F. et al. Raman and SERS microscopy for molecular imaging of live cells. Nat. Protoc. 8, 677–692 (2013).
Matousek, P. et al. Subsurface probing in diffusely scattering media using spatially offset Raman spectroscopy. Appl. Spectrosc. 59, 393–400 (2005).
Stone, N. et al. Surface enhanced spatially offset Raman spectroscopic (SESORS) imaging - the next dimension. Chem. Sci. 2, 776–780 (2011).
Butler, H.J. et al. Using Raman spectroscopy to characterize biological materials. Nat. Protoc. 11, 664–687 (2016).
Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82, 70–77 (1959).
Jokerst, J.V., Lobovkina, T., Zare, R.N. & Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine (Lond.) 6, 715–728 (2011).
Jokerst, J.V., Miao, Z., Zavaleta, C., Cheng, Z. & Gambhir, S.S. Affibody-functionalized gold-silica nanoparticles for Raman molecular imaging of the epidermal growth factor receptor. Small 7, 625–633 (2011).
Cushing, S.K. et al. Photocatalytic activity enhanced by plasmonic resonant energy transfer from metal to semiconductor. J. Am. Chem. Soc. 134, 15033–15041 (2012).
Pandikumar, A. et al. Titania@gold plasmonic nanoarchitectures: an ideal photoanode for dye-sensitized solar cells. Renew. Sustain. Energy Rev. 60, 408–420 (2016).
Zhou, N. et al. Plasmon-enhanced light harvesting: applications in enhanced photocatalysis, photodynamic therapy and photovoltaics. RSC Adv. 5, 29076–29097 (2015).
Acknowledgements
We thank T. Shaffer (Department of Radiology, Stanford University) for verifying and reproducing our results using the described protocol. We further acknowledge the MSKCC Electron Microscopy and Molecular Cytology core facilities for technical support, and C. Andreou (MSKCC) for helpful suggestions. The following funding sources are acknowledged: NIH R01 EB017748 (M.F.K.); NIH K08 CA16396 (M.F.K.); a Pershing Square Sohn Prize by the Pershing Square Sohn Cancer Research Alliance (M.F.K.); The Dana Foundation Brain and Immuno-Imaging Grant (M.F.K.); a Dana Neuroscience Scholar Award (M.F.K.); an MSKCC Brain Tumor Center Grant (M.F.K.); an MSKCC Center for Molecular Imaging and Nanotechnology Grant (M.F.K.); an MSKCC Technology Development Grant (M.F.K.); a grant from the 'Mr. William H. and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research'; a grant from 'The Center for Experimental Therapeutics Center of Memorial Sloan Kettering Cancer Center' (M.F.K.); a Geoffrey Beene Cancer Research Center at MSKCC Grant and Shared Resources Award (M.F.K.); a RSNA Research Scholar Grant (M.F.K.); a Society of MSKCC Research Grant (M.F.K.); and a grant from the National Natural Science Foundation (81401461; R.H.). We also acknowledge the grant-funding support provided by the MSKCC NIH Core Grant (P30-CA008748). M.F.K. is a Damon Runyon-Rachleff Innovator supported (in part) by the Damon Runyon Cancer Research Foundation (DRR-29-14).
Author information
Authors and Affiliations
Contributions
S.H. and M.A.W. designed the protocol, performed the experiments and wrote the paper. R.H. performed the animal experiments and tissue processing, and wrote the paper. M.F.K. supervised the researchers and edited the paper.
Corresponding author
Ethics declarations
Competing interests
S.H., M.A.W. and M.F.K. have filed patent applications focused on SERRS nanoparticle composition and synthesis methods, and Raman detection hardware. M.F.K. is a cofounder of the startup company RIO Imaging, which has licensed these patents.
Rights and permissions
About this article
Cite this article
Harmsen, S., Wall, M., Huang, R. et al. Cancer imaging using surface-enhanced resonance Raman scattering nanoparticles. Nat Protoc 12, 1400–1414 (2017). https://doi.org/10.1038/nprot.2017.031
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2017.031
This article is cited by
-
In vivo imaging using surface enhanced spatially offset raman spectroscopy (SESORS): balancing sampling frequency to improve overall image acquisition
npj Imaging (2024)
-
Molecular imaging: design mechanism and bioapplications
Science China Chemistry (2023)
-
Low-dose X-ray enhanced tumor accumulation of theranostic nanoparticles for high-performance bimodal imaging-guided photothermal therapy
Journal of Nanobiotechnology (2021)
-
Accelerated mapping of electronic density of states patterns of metallic nanoparticles via machine-learning
Scientific Reports (2021)
-
Cargo loading within ferritin nanocages in preparation for tumor-targeted delivery
Nature Protocols (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.