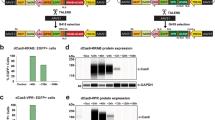
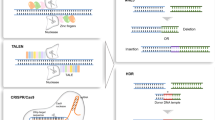
Abstract
Targeted nucleases, including zinc-finger nucleases (ZFNs), transcription activator-like (TAL) effector nucleases (TALENs) and clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated protein 9 (Cas9), have provided researchers with the ability to manipulate nearly any genomic sequence in human cells and model organisms. However, realizing the full potential of these genome-modifying technologies requires their safe and efficient delivery into relevant cell types. Unlike methods that rely on expression from nucleic acids, the direct delivery of nuclease proteins to cells provides rapid action and fast turnover, leading to fewer off-target effects while maintaining high rates of targeted modification. These features make nuclease protein delivery particularly well suited for precision genome engineering. Here we describe procedures for implementing protein-based genome editing in human embryonic stem cells and primary cells. Protocols for the expression, purification and delivery of ZFN proteins, which are intrinsically cell-permeable; TALEN proteins, which can be internalized via conjugation with cell-penetrating peptide moieties; and Cas9 ribonucleoprotein, whose nucleofection into cells facilitates rapid induction of multiplexed modifications, are described, along with procedures for evaluating nuclease protein activity. Once they are constructed, nuclease proteins can be expressed and purified within 6 d, and they can be used to induce genomic modifications in human cells within 2 d.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Gaj, T., Gersbach, C.A. & Barbas, C.F. III. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 31, 397–405 (2013).
Carroll, D. Genome engineering with targetable nucleases. Annu. Rev. Biochem. 83, 409–439 (2014).
Kim, H. & Kim, J.S. A guide to genome engineering with programmable nucleases. Nat. Rev. Genet. 15, 321–334 (2014).
Rouet, P., Smih, F. & Jasin, M. Expression of a site-specific endonuclease stimulates homologous recombination in mammalian cells. Proc. Natl. Acad. Sci. USA 91, 6064–6068 (1994).
Bibikova, M., Golic, M., Golic, K.G. & Carroll, D. Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics 161, 1169–1175 (2002).
Santiago, Y. et al. Targeted gene knockout in mammalian cells by using engineered zinc-finger nucleases. Proc. Natl. Acad. Sci. USA 105, 5809–5814 (2008).
Porteus, M.H. & Baltimore, D. Chimeric nucleases stimulate gene targeting in human cells. Science 300, 763 (2003).
Bibikova, M., Beumer, K., Trautman, J.K. & Carroll, D. Enhancing gene targeting with designed zinc-finger nucleases. Science 300, 764 (2003).
Urnov, F.D. et al. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature 435, 646–651 (2005).
Kim, Y.G., Cha, J. & Chandrasegaran, S. Hybrid restriction enzymes: zinc finger fusions to FokI cleavage domain. Proc. Natl. Acad. Sci. USA 93, 1156–1160 (1996).
Miller, J.C. et al. A TALE nuclease architecture for efficient genome editing. Nat. Biotechnol. 29, 143–148 (2011).
Wolfe, S.A., Nekludova, L. & Pabo, C.O. DNA recognition by Cys2His2 zinc finger proteins. Annu. Rev. Biophys. Biomol. Struct. 29, 183–212 (2000).
Gersbach, C.A., Gaj, T. & Barbas, C.F. III. Synthetic zinc finger proteins: the advent of targeted gene regulation and genome modification technologies. Acc. Chem. Res. 47, 2309–2318 (2014).
Boch, J. et al. Breaking the code of DNA binding specificity of TAL-type III effectors. Science 326, 1509–1512 (2009).
Moscou, M.J. & Bogdanove, A.J. A simple cipher governs DNA recognition by TAL effectors. Science 326, 1501 (2009).
Cermak, T. et al. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 39, e82 (2011).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Jinek, M. et al. RNA-programmed genome editing in human cells. Elife 2, e00471 (2013).
Cho, S.W., Kim, S., Kim, J.M. & Kim, J.S. Targeted genome engineering in human cells with the Cas9 RNA-guided endonuclease. Nat. Biotechnol. 31, 230–232 (2013).
Maggio, I. & Goncalves, M.A. Genome editing at the crossroads of delivery, specificity, and fidelity. Trends Biotechnol. 33, 280–291 (2015).
Ain, Q.U., Chung, J.Y. & Kim, Y.H. Current and future delivery systems for engineered nucleases: ZFN, TALEN and RGEN. J. Control. Release 205, 120–127 (2015).
Chu, G., Hayakawa, H. & Berg, P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 15, 1311–1326 (1987).
Hamm, A., Krott, N., Breibach, I., Blindt, R. & Bosserhoff, A.K. Efficient transfection method for primary cells. Tissue Eng. 8, 235–245 (2002).
Chen, C.A. & Okayama, H. Calcium phosphate-mediated gene transfer: a highly efficient transfection system for stably transforming cells with plasmid DNA. Biotechniques 6, 632–638 (1988).
Felgner, P.L. et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA 84, 7413–7417 (1987).
Boussif, O. et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl. Acad. Sci. USA 92, 7297–7301 (1995).
Eguchi, A. et al. Protein transduction domain of HIV-1 Tat protein promotes efficient delivery of DNA into mammalian cells. J. Biol. Chem. 276, 26204–26210 (2001).
Torchilin, V.P. et al. Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes. Proc. Natl. Acad. Sci. USA 100, 1972–1977 (2003).
Mello de Queiroz, F., Sanchez, A., Agarwal, J.R., Stuhmer, W. & Pardo, L.A. Nucleofection induces non-specific changes in the metabolic activity of transfected cells. Mol. Biol. Rep. 39, 2187–2194 (2012).
Maurisse, R. et al. Comparative transfection of DNA into primary and transformed mammalian cells from different lineages. BMC Biotechnol. 10, 9 (2010).
Li, H. et al. In vivo genome editing restores haemostasis in a mouse model of haemophilia. Nature 475, 217–221 (2011).
Anguela, X.M. et al. Robust ZFN-mediated genome editing in adult hemophilic mice. Blood 122, 3283–3287 (2013).
Ran, F.A. et al. In vivo genome editing using Staphylococcus aureus Cas9. Nature 520, 186–191 (2015).
Swiech, L. et al. In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat. Biotechnol. 33, 102–106 (2015).
Asuri, P. et al. Directed evolution of adeno-associated virus for enhanced gene delivery and gene targeting in human pluripotent stem cells. Mol. Ther. 20, 329–338 (2012).
Handel, E.M. et al. Versatile and efficient genome editing in human cells by combining zinc-finger nucleases with adeno-associated viral vectors. Hum. Gene Ther. 23, 321–329 (2012).
Ellis, B.L., Hirsch, M.L., Porter, S.N., Samulski, R.J. & Porteus, M.H. Zinc-finger nuclease-mediated gene correction using single AAV vector transduction and enhancement by Food and Drug Administration–approved drugs. Gene Ther. 20, 35–42 (2013).
Senis, E. et al. CRISPR/Cas9-mediated genome engineering: an adeno-associated viral (AAV) vector toolbox. Biotechnol. J. 9, 1402–1412 (2014).
Lombardo, A. et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat. Biotechnol. 25, 1298–1306 (2007).
Joglekar, A.V. et al. Integrase-defective lentiviral vectors as a delivery platform for targeted modification of adenosine deaminase locus. Mol. Ther. 21, 1705–1717 (2013).
Kabadi, A.M., Ousterout, D.G., Hilton, I.B. & Gersbach, C.A. Multiplex CRISPR/Cas9-based genome engineering from a single lentiviral vector. Nucleic Acids Res. 42, e147 (2014).
Heckl, D. et al. Generation of mouse models of myeloid malignancy with combinatorial genetic lesions using CRISPR-Cas9 genome editing. Nat. Biotechnol. 32, 941–946 (2014).
Shalem, O. et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87 (2014).
Wang, T., Wei, J.J., Sabatini, D.M. & Lander, E.S. Genetic screens in human cells using the CRISPR-Cas9 system. Science 343, 80–84 (2014).
Gilbert, L.A. et al. Genome-scale CRISPR-mediated control of gene repression and activation. Cell 159, 647–661 (2014).
Holkers, M. et al. Differential integrity of TALE nuclease genes following adenoviral and lentiviral vector gene transfer into human cells. Nucleic Acids Res. 41, e63 (2013).
Mock, U. et al. Novel lentiviral vectors with mutated reverse transcriptase for mRNA delivery of TALE nucleases. Sci. Rep. 4, 6409 (2014).
Kim, Y. et al. A library of TAL effector nucleases spanning the human genome. Nat. Biotechnol. 31, 251–258 (2013).
Perez, E.E. et al. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat. Biotechnol. 26, 808–816 (2008).
Maggio, I. et al. Adenoviral vector delivery of RNA-guided CRISPR/Cas9 nuclease complexes induces targeted mutagenesis in a diverse array of human cells. Sci. Rep. 4, 5105 (2014).
Ding, Q. et al. Permanent alteration of PCSK9 with in vivo CRISPR-Cas9 genome editing. Circ. Res. 115, 488–492 (2014).
Cheng, R. et al. Efficient gene editing in adult mouse livers via adenoviral delivery of CRISPR/Cas9. FEBS Lett. 588, 3954–3958 (2014).
Holkers, M. et al. Adenoviral vector DNA for accurate genome editing with engineered nucleases. Nat. Methods 11, 1051–1057 (2014).
Bobis-Wozowicz, S. et al. Non-integrating gamma-retroviral vectors as a versatile tool for transient zinc-finger nuclease delivery. Sci. Rep. 4, 4656 (2014).
Phang, R.Z. et al. Zinc finger nuclease-expressing baculoviral vectors mediate targeted genome integration of reprogramming factor genes to facilitate the generation of human induced pluripotent stem cells. Stem Cells Transl. Med. 2, 935–945 (2013).
Zhu, H. et al. Baculoviral transduction facilitates TALEN-mediated targeted transgene integration and Cre/LoxP cassette exchange in human-induced pluripotent stem cells. Nucleic Acids Res. 41, e180 (2013).
Tay, F.C. et al. Targeted transgene insertion into the AAVS1 locus driven by baculoviral vector-mediated zinc finger nuclease expression in human-induced pluripotent stem cells. J. Gene Med. 15, 384–395 (2013).
Lau, C.H. et al. Genetic rearrangements of variable di-residue (RVD)-containing repeat arrays in a baculoviral TALEN system. Mol. Ther. Methods Clin. Dev. 1, 14050 (2014).
Pruett-Miller, S.M., Reading, D.W., Porter, S.N. & Porteus, M.H. Attenuation of zinc finger nuclease toxicity by small-molecule regulation of protein levels. PLoS Genet. 5, e1000376 (2009).
Gaj, T., Guo, J., Kato, Y., Sirk, S.J. & Barbas, C.F. III Targeted gene knockout by direct delivery of zinc-finger nuclease proteins. Nat. Methods 9, 805–807 (2012).
Hendel, A., Fine, E.J., Bao, G. & Porteus, M.H. Quantifying on- and off-target genome editing. Trends Biotechnol. 33, 132–140 (2015).
Liu, J., Gaj, T., Wallen, M.C. & Barbas, C.F. III. Improved cell-penetrating zinc-finger nuclease proteins for precision genome engineering. Mol. Ther. Nucleic Acids 4, e232 (2015).
Song, Y. et al. Expression, purification and characterization of zinc-finger nuclease to knockout the goat beta-lactoglobulin gene. Protein Expr. Purif. 112, 1–7 (2015).
Gaj, T., Liu, J., Anderson, K.E., Sirk, S.J. & Barbas, C.F. III. Protein delivery using Cys2-His2 zinc-finger domains. ACS Chem. Biol. 9, 1662–1667 (2014).
Gaj, T. & Liu, J. Direct protein delivery to mammalian cells using cell-permeable Cys2-His2 zinc-finger domains. J. Vis. Exp. doi:10.3791/52814 (2015).
Chen, Z. et al. Receptor-mediated delivery of engineered nucleases for genome modification. Nucleic Acids Res. 41, e182 (2013).
Cai, Y., Bak, R.O. & Mikkelsen, J.G. Targeted genome editing by lentiviral protein transduction of zinc-finger and TAL-effector nucleases. Elife 3, e01911 (2014).
Liu, J., Gaj, T., Patterson, J.T., Sirk, S.J. & Barbas, C.F. III. Cell-penetrating peptide-mediated delivery of TALEN proteins via bioconjugation for genome engineering. PLoS ONE 9, e85755 (2014).
Ru, R. et al. Targeted genome engineering in human induced pluripotent stem cells by penetrating TALENs. Cell Regen. 2, 5–12 (2013).
Kim, S., Kim, D., Cho, S.W., Kim, J. & Kim, J.S. Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 24, 1012–1019 (2014).
Lin, S., Staahl, B.T., Alla, R.K. & Doudna, J.A. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. Elife 3, e04766 (2014).
Zuris, J.A. et al. Cationic lipid-mediated delivery of proteins enables efficient protein-based genome editing in vitro and in vivo. Nat. Biotechnol. 33, 73–80 (2015).
Ramakrishna, S. et al. Gene disruption by cell-penetrating peptide-mediated delivery of Cas9 protein and guide RNA. Genome Res. 24, 1020–1027 (2014).
D'Astolfo, D.S. et al. Efficient intracellular delivery of native proteins. Cell 161, 674–690 (2015).
Doyon, Y. et al. Transient cold shock enhances zinc-finger nuclease-mediated gene disruption. Nat. Methods 7, 459–460 (2010).
Chen, F. et al. High-frequency genome editing using ssDNA oligonucleotides with zinc-finger nucleases. Nat. Methods 8, 753–755 (2011).
Olson, B.J. & Markwell, J. Assays for determination of protein concentration. Curr. Protoc. Protein Sci. 48, 3.4.1–3.4.29 (2007).
Walker, J.M. The bicinchoninic acid (BCA) assay for protein quantitation. Methods Mol. Biol. 32, 5–8 (1994).
Kruger, N.J. The Bradford method for protein quantitation. Methods Mol. Biol. 32, 9–15 (1994).
Pruett-Miller, S.M. & Davis, G.D. Donor plasmid design for codon and single base genome editing using zinc finger nucleases. Methods Mol. Biol. 1239, 219–229 (2015).
Hockemeyer, D. et al. Efficient targeting of expressed and silent genes in human ESCs and iPSCs using zinc-finger nucleases. Nat. Biotechnol. 27, 851–857 (2009).
Guo, J., Gaj, T. & Barbas, C.F. III. Directed evolution of an enhanced and highly efficient FokI cleavage domain for zinc finger nucleases. J. Mol. Biol. 400, 96–107 (2010).
Segal, D.J., Crotty, J.W., Bhakta, M.S., Barbas, C.F. III & Horton, N.C. Structure of Aart, a designed six-finger zinc finger peptide, bound to DNA. J. Mol. Biol. 363, 405–421 (2006).
Mak, A.N., Bradley, P., Cernadas, R.A., Bogdanove, A.J. & Stoddard, B.L. The crystal structure of TAL effector PthXo1 bound to its DNA target. Science 335, 716–719 (2012).
Nishimasu, H. et al. Crystal structure of Cas9 in complex with guide RNA and target DNA. Cell 156, 935–949 (2014).
Wingfield, P.T. Preparation of soluble proteins from Escherichia coli. Curr. Protoc. Protein Sci. 78, 6.2.1–6.2.22 (2014).
Guschin, D.Y. et al. A rapid and general assay for monitoring endogenous gene modification. Methods Mol. Biol. 649, 247–256 (2010).
Bergkessel, M. & Guthrie, C. Colony PCR. Methods Enzymol. 529, 299–309 (2013).
Gonzalez, B. et al. Modular system for the construction of zinc-finger libraries and proteins. Nat. Protoc. 5, 791–810 (2010).
Kim, H.J., Lee, H.J., Kim, H., Cho, S.W. & Kim, J.S. Targeted genome editing in human cells with zinc finger nucleases constructed via modular assembly. Genome Res. 19, 1279–1288 (2009).
Maeder, M.L., Thibodeau-Beganny, S., Sander, J.D., Voytas, D.F. & Joung, J.K. Oligomerized pool engineering (OPEN): an 'open-source' protocol for making customized zinc-finger arrays. Nat. Protoc. 4, 1471–1501 (2009).
Kim, S., Lee, M.J., Kim, H., Kang, M. & Kim, J.S. Preassembled zinc-finger arrays for rapid construction of ZFNs. Nat. Methods 8, 7 (2011).
Bhakta, M.S. et al. Highly active zinc-finger nucleases by extended modular assembly. Genome Res. 23, 530–538 (2013).
Gupta, A. et al. An optimized two-finger archive for ZFN-mediated gene targeting. Nat. Methods 9, 588–590 (2012).
Sander, J.D. et al. Selection-free zinc-finger-nuclease engineering by context-dependent assembly (CoDA). Nat. Methods 8, 67–69 (2011).
Sanjana, N.E. et al. A transcription activator-like effector toolbox for genome engineering. Nat. Protoc. 7, 171–192 (2012).
Reyon, D. et al. FLASH assembly of TALENs for high-throughput genome editing. Nat. Biotechnol. 30, 460–465 (2012).
Schmid-Burgk, J.L., Schmidt, T., Kaiser, V., Honing, K. & Hornung, V. A ligation-independent cloning technique for high-throughput assembly of transcription activator-like effector genes. Nat. Biotechnol. 31, 76–81 (2013).
Ran, F.A. et al. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 (2013).
Acknowledgements
We thank S.J. Sirk for critical reading of the manuscript. This work was supported by the National Institutes of Health (DP1CA174426 to C.F.B.), The Skaggs Institute for Chemical Biology (to C.F.B.), the Institute for Basic Science (IBS-R021-D1 to J.-S.K.) and ShanghaiTech University (to J.L.). Molecular graphics were generated by PyMol.
Author information
Authors and Affiliations
Contributions
J.L., T.G. and C.F.B. conceived the study; J.L., T.G., C.F.B. and J.-S.K. designed the research; J.L., Y.Y., N.W., S.S., S.K. and C.N.K. performed the experiments; and T.G., J.L. and J.-S.K. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
J.-S.K. is a co-founder of ToolGen.
Rights and permissions
About this article
Cite this article
Liu, J., Gaj, T., Yang, Y. et al. Efficient delivery of nuclease proteins for genome editing in human stem cells and primary cells. Nat Protoc 10, 1842–1859 (2015). https://doi.org/10.1038/nprot.2015.117
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2015.117
This article is cited by
-
Gene-Modified Stem Cells for Spinal Cord Injury: a Promising Better Alternative Therapy
Stem Cell Reviews and Reports (2022)
-
Semi-Implantable Bioelectronics
Nano-Micro Letters (2022)
-
CRISPR-based strategies in infectious disease diagnosis and therapy
Infection (2021)
-
Integrating gene delivery and gene-editing technologies by adenoviral vector transfer of optimized CRISPR-Cas9 components
Gene Therapy (2020)
-
A biodegradable nanocapsule delivers a Cas9 ribonucleoprotein complex for in vivo genome editing
Nature Nanotechnology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.