Abstract
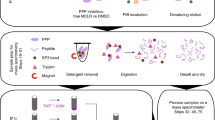
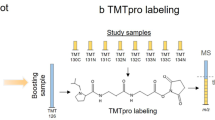
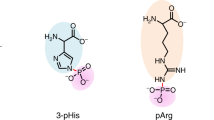
The characterization of phosphorylated proteins is a challenging analytical task since many of the proteins targeted for phosphorylation are low in abundance and phosphorylation is typically substoichiometric. Highly efficient enrichment procedures are therefore required. Here we describe a protocol for selective phosphopeptide enrichment using titanium dioxide (TiO2) chromatography. The selectivity toward phosphopeptides is obtained by loading the sample in a 2,5-dihydroxybenzoic acid (DHB) or phthalic acid solution containing acetonitrile and trifluoroacetic acid (TFA) onto a TiO2 micro-column. Although phosphopeptide enrichment can be achieved by using TFA and acetonitrile alone, the selectivity is dramatically enhanced by adding DHB or phthalic acid since these compounds, in conjunction with the low pH caused by TFA, prevent binding of nonphosphorylated peptides to TiO2. Using an alkaline solution (pH ≥ 10.5) both monophosphorylated and multiphosphorylated peptides are eluted from the TiO2 beads. This highly efficient method for purification of phosphopeptides is well suited for the characterization of phosphoproteins from both in vitro and in vivo studies in combination with mass spectrometry (MS). It is a very easy and fast method. The entire protocol requires less than 15 min per sample if the buffers have been prepared in advance (not including lyophilization).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Graves, J.D. & Krebs, E.G. Protein phosphorylation and signal transduction. Pharmacol. Ther. 82, 111–121 (1999).
Manning, G., Whyte, D.B., Martinez, R., Hunter, T. & Sudarsanam, S. The protein kinase complement of the human genome. Science 298, 1912–1934 (2002).
Venter, J.C. et al. The sequence of the human genome. Science 291, 1304–1351 (2001).
Hubbard, M.J. & Cohen, P. On target with a new mechanism for the regulation of protein-phosphorylation. Trends Biochem. Sci. 18, 172–177 (1993).
Gronborg, M. et al. A mass spectrometry–based proteomic approach for identification of serine/threonine-phosphorylated proteins by enrichment with phospho-specific antibodies: identification of a novel protein, Frigg, as a protein kinase A substrate. Mol. Cell. Proteomics 1, 517–527 (2002).
Pandey, A. et al. Identification of a novel immunoreceptor tyrosine-based activation motif-containing molecule, STAM2, by mass spectrometry and its involvement in growth factor and cytokine receptor signaling pathways. J. Biol. Chem. 275, 38633–38639 (2000).
Guy, G.R., Philip, R. & Tan, Y.H. Analysis of cellular phosphoproteins by two-dimensional gel electrophoresis: applications for cell signaling in normal and cancer cells. Electrophoresis 15, 417–440 (1994).
McLachlin, D.T. & Chait, B.T. Analysis of phosphorylated proteins and peptides by mass spectrometry. Curr. Opin. Chem. Biol. 5, 591–602 (2001).
Zhang, X. et al. Identification of phosphorylation sites in proteins separated by polyacrylamide gel electrophoresis. Anal. Chem. 70, 2050–2059 (1998).
Larsen, M.R., Sorensen, G.L., Fey, S.J., Larsen, P.M. & Roepstorff, P. Phospho-proteomics: evaluation of the use of enzymatic de-phosphorylation and differential mass spectrometric peptide mass mapping for site specific phosphorylation assignment in proteins separated by gel electrophoresis. Proteomics 1, 223–238 (2001).
Gruhler, A. et al. Quantitative phosphoproteomics applied to the yeast pheromone signaling pathway. Mol. Cell. Proteomics 4, 310–327 (2005).
Chalmers, M.J. et al. Protein kinase A phosphorylation characterized by tandem Fourier transform ion cyclotron resonance mass spectrometry. Proteomics 4, 970–981 (2004).
Schroeder, M.J., Webb, D.J., Shabanowitz, J., Horwitz, A.F. & Hunt, D.F. Methods for the detection of paxillin post-translational modifications and interacting proteins by mass spectrometry. J. Proteome Res. 4, 1832–41 (2005).
Carr, S.A., Huddleston, M.J. & Annan, R.S. Selective detection and sequencing of phosphopeptides at the femtomole level by mass spectrometry. Anal. Biochem. 239, 180–192 (1996).
Bateman, R.H. et al. A novel precursor ion discovery method on a hybrid quadrupole orthogonal acceleration time-of-flight (Q-TOF) mass spectrometer for studying protein phosphorylation. J. Am. Soc. Mass Spectrom. 13, 792–803 (2002).
Steen, H., Jebanathirajah, J.A., Rush, J., Morrice, N. & Kirschner, M.W. Phosphorylation analysis by mass spectrometry: myths, facts, and the consequences for qualitative and quantitative measurements. Mol. Cell. Proteomics 5, 172–181 (2006).
Kjellstrom, S. & Jensen, O.N. Phosphoric acid as a matrix additive for MALDI MS analysis of phosphopeptides and phosphoproteins. Anal. Chem. 76, 5109–5117 (2004).
Asara, J.M. & Allison, J. Enhanced detection of phosphopeptides in matrix-assisted laser desorption/ionization mass spectrometry using ammonium salts. J. Am. Soc. Mass Spectrom. 10, 35–44 (1999).
Beausoleil, S.A. et al. Large-scale characterization of HeLa cell nuclear phosphoproteins. Proc. Natl. Acad. Sci. USA 101, 12130–12135 (2004).
Nuhse, T.S., Stensballe, A., Jensen, O.N. & Peck, S.C. Large-scale analysis of in vivo phosphorylated membrane proteins by immobilized metal ion affinity chromatography and mass spectrometry. Mol. Cell. Proteomics 2, 1234–1243 (2003).
Ficarro, S.B. et al. Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae. Nat. Biotechnol. 20, 301–305 (2002).
Smith, J.C. & Figeys, D. Proteomics technology in systems biology. Mol. BioSyst. 2, 364–370 (2006).
Stewart, Thomson, T. & Figeys, D. O-18 Labeling: a tool for proteomics. Rapid Commun. Mass Spectrom. 15, 2456–2465 (2001).
Speicher, K.D., Kolbas, O., Harper, S. & Speicher, D.W. Systematic analysis of peptide recoveries from in-gel digestions for protein identifications in proteome studies. J. Biomol. Tech. 11, 74–86 (2000).
Oda, Y., Nagasu, T. & Chait, B.T. Enrichment analysis of phosphorylated proteins as a tool for probing the phosphoproteome. Nat. Biotechnol. 19, 379–382 (2001).
McLachlin, D.T. & Chait, B.T. Improved beta-elimination-based affinity purification strategy for enrichment of phosphopeptides. Anal. Chem. 75, 6826–6836 (2003).
Pinkse, M.W.H., Uitto, P.M., Hilhorst, M.J., Ooms, B. & Heck, A.J.R. Selective isolation at the femtomole level of phosphopeptides from proteolytic digests using 2D-nanoLC-ESI-MS/MS and titanium oxide precolumns. Anal. Chem. 76, 3935–3943 (2004).
Larsen, M.R., Thingholm, T.E., Jensen, O.N., Roepstorff, P. & Jørgensen, T.J.D. Highly selective enrichment of phosphorylated peptides from peptide mixtures using titanium dioxide microcolumns. Mol. Cell. Proteomics 4, 873–886 (2005).
Larsen, M.R., Graham, M., Robinson, P.J. & Roepstorff, P. Improved mass spectrometric detection of hydrophilic phosphopeptides using graphite powder micro-columns. Mol. Cell. Proteomics 3, 456–465 (2004).
Rinalducci, S., Larsen, M.R., Mohammed, S. & Zolla, L. Novel protein phosphorylation site identification in spinach stroma membranes by titanium dioxide microcolumns and tandem mass spectrometry. J. Proteome Res. 5, 973–982 (2006).
Rappsilber, J., Ishihama, Y. & Mann, M. Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal. Chem. 75, 663–670 (2003).
Gobom, J., Nordhoff, E., Mirgorodskaya, E., Ekman, R. & Roepstorff, P. Sample purification and preparation technique based on nano-scale reversed-phase columns for the sensitive analysis of complex peptide mixtures by matrix-assisted laser desorption/ionization mass spectrometry. J. Mass Spectrom. 34, 105–116 (1999).
Liu, S. et al. Formation of phosphopeptide-metal ion complexes in liquid chromatography/electrospray mass spectrometry and their influence on phosphopeptide detection. Rapid Commun. Mass Spectrom. 19, 2747–2756 (2005).
Acknowledgements
This work was supported by the Danish Natural Science Research Council (grant 21-03-0167 to M.R.L.) and the Danish Strategic Research Council (to O.N.J.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Thingholm, T., Jørgensen, T., Jensen, O. et al. Highly selective enrichment of phosphorylated peptides using titanium dioxide. Nat Protoc 1, 1929–1935 (2006). https://doi.org/10.1038/nprot.2006.185
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2006.185
This article is cited by
-
Preosteoclast plays a pathogenic role in syndesmophyte formation of ankylosing spondylitis through the secreted PDGFB — GRB2/ERK/RUNX2 pathway
Arthritis Research & Therapy (2023)
-
Phosphoproteomic analysis on ovarian follicles reveals the involvement of LSD1 phosphorylation in Chicken follicle selection
BMC Genomics (2023)
-
Phosphorylation of β-catenin at Serine552 correlates with invasion and recurrence of non-functioning pituitary neuroendocrine tumours
Acta Neuropathologica Communications (2022)
-
ATR and CDK4/6 inhibition target the growth of methotrexate-resistant choriocarcinoma
Oncogene (2022)
-
Global identification of phospho-dependent SCF substrates reveals a FBXO22 phosphodegron and an ERK-FBXO22-BAG3 axis in tumorigenesis
Cell Death & Differentiation (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.