Abstract
(±)Modafinil ((±)MOD) and its R-enantiomer (R-modafinil; R-MOD) have been investigated for their potential as treatments for psychostimulant addiction. We recently reported a series of (±)MOD analogs, of which JJC8-016 (N-(2-((bis(4-fluorophenyl)methyl)thio)ethyl)-3-phenylpropan-1-amine) was selected for further development. JJC8-016 and R-MOD were evaluated for binding across ~70 receptors, transporters, and enzymes. Although at a concentration of 10 μM, there were many hits for JJC8-016, binding affinities in the range of its DAT affinity were only observed at the serotonin transporter (SERT), dopamine D2-like, and sigma1 receptors. R-MOD was more selective, but had much lower affinity at the DAT (Ki=3 μM) than JJC8-016 (Ki=116 nM). In rats, systemic administration of R-MOD alone (10–30 mg/kg i.p.) dose-dependently increased locomotor activity and electrical brain-stimulation reward, whereas JJC8-016 (10–30 mg/kg i.p.) did not produce these effects. Strikingly, pretreatment with JJC8-016 dose-dependently inhibited cocaine-enhanced locomotion, cocaine self-administration, and cocaine-induced reinstatement of drug-seeking behavior, whereas R-MOD inhibited cocaine-induced reinstatement only at the high dose of 100 mg/kg. Notably, JJC8-016 alone neither altered extracellular dopamine in the nucleus accumbens nor maintained self-administration. It also failed to induce reinstatement of drug-seeking behavior. These findings suggest that JJC8-016 is a unique DAT inhibitor that has no cocaine-like abuse potential by itself. Moreover, pretreatment with JJC8-016 significantly inhibits cocaine-taking and cocaine-seeking behavior likely by interfering with cocaine binding to DAT. In addition, off-target actions may also contribute to its potential therapeutic utility in the treatment of cocaine abuse.
Similar content being viewed by others
Introduction
Cocaine addiction continues to be a serious public health problem and, so far, no effective pharmacotherapies are available for treatment. The mesolimbic dopamine (DA) pathway, which originates in the midbrain ventral tegmental area (VTA) and projects to the nucleus accumbens (NAc) and prefrontal cortex (PFC), is believed to be essential for both drug reward and relapse to drug-seeking behavior (Luscher and Malenka, 2011; Swanson, 1982; Wise et al, 1996). As cocaine significantly enhances extracellular DA by inhibiting the reuptake of DA via the DA transporter (DAT), pharmacological blockade of DAT has been proposed as a mechanism to treat cocaine addiction (Di Chiara and Imperato, 1988; Hiranita et al, 2009; Tanda et al, 2009; Torregrossa and Kalivas, 2008; Xi et al, 2016). Accordingly, many DAT inhibitors have been developed and several of them such as GBR 12909 and methylphenidate have been examined for treatment of cocaine use disorders (Howell and Wilcox, 2001; Newman and Kulkarni, 2002; Platt et al, 2002; Rothman et al, 2008; Rothman and Glowa, 1995; Runyon and Carroll, 2006). However, none have proven successful because of their potential side effects, for example, abuse liability and/or significant cardiovascular actions (Howell and Wilcox, 2001; Rothman et al, 2008). Therefore, development of DAT inhibitors for human use has been stymied (Czoty et al, 2016). One approach toward a medication strategy has been to develop novel atypical DAT inhibitors that bind to the DAT, thus not only preventing cocaine from binding and rapidly inhibiting DA uptake, but also demonstrating less cocaine-like abuse potential (Reith et al, 2015).
(±)Modafinil ((±)MOD) is an FDA-approved treatment for narcolepsy and other sleep disorders (Ballon and Feifel, 2006; Garnock-Jones et al, 2009). (±)MOD has been described as a mild psychostimulant without abuse liability and is also used off label for the treatment of cognitive disorders (Minzenberg and Carter, 2008; Schmaal et al, 2013). (±)MOD has been promoted as a potential treatment for psychostimulant dependence because of its different binding property to the DAT from cocaine (Cao et al, 2010; Madras et al, 2006; Mereu et al, 2013, 2017; Mignot et al, 1994; Zolkowska et al, 2009). (±)MOD was first found to be an effective treatment for amphetamine dependence in patients with comorbid social phobia (Camacho and Stein, 2002). This finding was later supported by a number of studies demonstrating that (±)MOD was effective in reducing cocaine and methamphetamine use, subjective euphoric effects, craving, and withdrawal symptoms (Dackis et al, 2003, 2005; De La Garza et al, 2010; Goudriaan et al, 2013; Hart et al, 2008; Kampman et al, 2015; Malcolm et al, 2006; McGregor et al, 2008; Shearer et al, 2009; Verrico et al, 2014), although several others failed to confirm the therapeutic efficacy of (±)MOD in treatment of cocaine (Dackis et al, 2012) or methamphetamine dependence in larger cohorts (Anderson et al, 2012; Heinzerling et al, 2010). In experimental animals, (±)MOD has been reported to block reinstatement of drug seeking caused by cocaine, heroin, methamphetamine, or drug-associated environmental cues (Mahler et al, 2014; Reichel and See, 2010, 2012; Tahsili-Fahadan et al, 2010).
(±)MOD, by definition, comprises the R-(−)- and S-(+)-enantiomers. R-MOD is approximately threefold more potent than its S-enantiomer at blocking DA uptake at the DAT (Loland et al, 2012). R-MOD is also metabolically more stable than S-MOD, and therefore has a longer half-life and improved pharmacokinetic profile (Robertson and Hellriegel, 2003; Wong et al, 1999). We have reported that R-MOD is more potent and effective than its S-enantiomer in attenuating nicotine self-administration, and nicotine- or cue-induced reinstatement of drug-seeking behavior in Long–Evans rats and in alcohol-preferring rats (Wang et al, 2015).
We recently developed a series of novel MOD analogs with higher affinity for the DAT than the parent compound (Okunola-Bakare et al, 2014). JJC8-016 (N-(2-((bis(4-fluorophenyl)methyl)thio)ethyl)-3-phenylpropan-1-amine) was selected as a lead compound as its affinity for the DAT (Ki=116 nM) and the serotonin transporter (SERT; Ki=360 nM) were higher than the parent drug, (±)MOD, and comparable to those of cocaine (Okunola-Bakare et al, 2014) (Table 1). We compared JJC8-016 with R-MOD at SERT, the D2-like, and sigma1 receptors, as previous structure–activity relationship studies in a series of both modafinil and benztropine-like molecules have shown overlap in binding affinities at these sites (Cao et al, 2016) (Table 1). Herein we extended these binding studies to a screen of ~70 receptors, transporters, and enzymes at concentrations of 100 nM and 10 μM. We then evaluated and compared the behavioral effects of both R-MOD and JJC8-016 in several rodent models of cocaine addiction. The results of these studies suggest that JJC8-016 may be more promising than R-MOD as a medication for the treatment of cocaine use disorders and that its unique pharmacological profile may provide leads for future drug development.
Materials and methods
Animals
Male Long–Evans rats (Charles River Laboratories, Raleigh, NC) were used. All rats were housed individually in a climate-controlled room under a 12 h light/dark cycle. Food and water were available ad libitum throughout the experiments. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals of the U.S. National Research Council and were approved by the animal care and use committee of the National Institute on Drug Abuse of the US National Institutes of Health.
Drugs
R-MOD and JJC8-016 were synthesized in the Medicinal Chemistry Section, National Institute on Drug Abuse–Intramural Research Program according to literature procedures (Cao et al, 2010; Okunola-Bakare et al, 2014). R-MOD and JJC8-016 were dissolved in sterile water containing 10% DMSO and 15% Tween-80 for intraperitoneal (i.p.) injection. In the intravenous (i.v.) JJC8-016 replacement experiment, JJC8-016 was dissolved in saline containing 1% DMSO and 1.5% Tween-80.
Experiment 1: In Vitro Screening
R-MOD was screened for binding to ~70 receptors, transporters, and enzymes at a single concentration of 100 μM and there were no ‘hits.’ JJC8-016 was tested in the same screens at concentrations of 100 and 10 000 nM. The results are displayed in Supplementary Tables S2 and S3.
Experiment 2: Cocaine Self-Administration
Intravenous cocaine self-administration
The procedures for jugular catheter surgery and cocaine self-administration were performed as previously reported (Le et al, 2006). The i.v. self-administration experiments were conducted in operant response test chambers from Med Associates (Georgia, VT). Each test chamber had an active lever and an inactive lever. Depression of the active lever activated the infusion pump; depression of the inactive lever was counted but had no consequence. After 7 days of recovery from surgery, rats were initially trained to self-administer cocaine (1.0 mg/kg/infusion) under FR1 reinforcement. Each cocaine infusion delivered a volume of 0.08 ml/infusion over 4.6 s and was paired with presentation of a stimulus light and tone. During the 4.6 s infusion time, additional responses on the active lever were recorded but did not lead to additional infusions. Each session lasted 3 h. FR1 reinforcement was used for 5–7 days. Then, subjects were allowed to continue cocaine (0.5 mg/kg/infusion) self-administration under FR2 reinforcement until stable cocaine self-administration was established: a minimum of 20 presses on the active lever per test session and stability criteria of <10% variability in interresponse interval, <10% variability in number of infusions taken, and <10% variability in number of presses on the active lever for at least 3 consecutive days. The dose of cocaine was chosen based on previous studies showing that 0.5 mg/kg/infusion of cocaine lies within the middle range of the descending limb of the cocaine dose–response self-administration curve, where reliable dose-dependent effects can be observed (Xi et al, 2005). In addition, we chose 0.5 mg/kg, rather than 1 mg/kg, of cocaine in order to increase the work demand (ie, lever presses) on the rats for the same amount of drug intake. In our experience, this approach increases the sensitivity of measuring changes in drug-seeking behavior. To avoid cocaine overdose during the self-administration period, each animal was limited to a maximum of 50 cocaine injections per 3 h session.
Effects of R-MOD and JJC8-016 on i.v. cocaine self-administration
The effects of R-MOD (10, 30, and 100 mg/kg) or JJC8-016 (10 and 30 mg/kg) 30 min before testing on cocaine self-administration were evaluated after stable cocaine self-administration was established for at least 3 consecutive days. After each test, rats then received an additional 3–5 days of self-administration of cocaine alone until stable self-administration was reestablished. The order of testing for the various doses of R-MOD and JJC8-018 was counterbalanced.
Drug substitution test
Two additional groups of rats were used to evaluate the addictive liability of JJC8-016. One group of rats was initially trained for JJC8-016 self-administration followed by cocaine substitution, and another group of rats was initially trained for cocaine self-administration followed by JJC8-016 substitution. The experimental procedures for JJC8-016 self-administration were the same as for cocaine self-administration except that different doses of JJC8-016 (1.0 mg/kg/infusion) and cocaine (0.5 mg/kg/infusion) were used. The dose of JJC8-016 was chosen based on the dose of R-MOD used in our previous self-administration and substitution tests (Wang et al, 2015) and the maximal solubility of JJC8-016 in the vehicle for i.v. drug administration. After stable cocaine self-administration was established for at least 3 consecutive days, the cocaine self-administration rats were switched to self-administer JJC8-016 (1.0 mg/kg/infusion). Similarly, JJC8-016 self-administration rats were switched to self-administer cocaine (0.5 mg/kg/infusion). As rats might take several days to support self-administration for a novel reinforcer, each replacement test was continued for at least 5 days.
Multiple-dose cocaine self-administration
To determine whether the pharmacological action of R-MOD or JJC8-016 was also cocaine dose dependent, we further studied the effects of R-MOD or JJC8-016 on cocaine self-administration maintained by a full range of cocaine doses (0.03, 0.06, 0.125, 0.25, 0.5, and 1.0 mg/kg/infusion) in a single session (Hiranita et al, 2009; Mantsch et al, 2007; Song et al, 2012). The session consisted of five sequential 20 min components, each preceded by a 20 min timeout period for changing cocaine dose. The infusion volumes and durations in each component were identical except that cocaine concentrations for corresponding unit cocaine doses differed. Training continued until: (1) a minimum of 5.0 mg/kg cocaine was self-administered within a session with <20% variation in the total number of cocaine injections compared with the previous session, (2) the dose of cocaine that maintained maximal response rates varied by no more than one-half log unit over two consecutive test sessions, and (3) maximal response rates were at least fivefold higher than response rates maintained during extinction. Then, each rat randomly received either vehicle or 1 of 2 doses of R-MOD or JJC8-016 (10 and 30 mg/kg, i.p.) 30 min before the test session. Rats then received an additional 3–4 days of self-administration of cocaine alone until the baseline response rate was reestablished before testing the next dose of R-MOD or JJC8-016. The order of testing for the various doses of drug or vehicle was counterbalanced.
Experiment 3: Oral Sucrose Self-Administration
The procedures for oral sucrose self-administration testing were identical to the procedures used for cocaine self-administration, except for the following: (1) no surgery was carried out in this experiment; (2) active lever presses led to delivery of 0.1 ml of 5% sucrose solution into a liquid food tray on the operant chamber wall; and (3) the maximal number of sucrose deliveries was 100. The effects of JJC8-016 (10 and 30 mg/kg, i.p.) on oral sucrose self-administration maintained under FR2 reinforcement were evaluated.
Experiment 4: Reinstatement to Cocaine-Seeking Behavior
After stable cocaine self-administration was established, rats were divided into four groups to evaluate the effects of R-MOD and JJC8-016 in the following experiments.
Cocaine-induced reinstatement
The extinction procedure was the same as described previously (Xi et al, 2006). During extinction, cocaine was replaced by saline, and the cocaine-associated cue-light and tone were turned off. Active lever-pressing led only to saline infusion. After the rats met the extinction criterion (⩽10 lever presses for 3 consecutive days), they were tested to study the effects of R-MOD (0, 10, 30, and 100 mg/kg, i.p.) or JJC8-016 (0, 10, and 30 mg/kg, i.p) on reinstatement of drug-seeking behavior induced by cocaine priming (10 mg/kg, i.p.).
R-MOD- or JJC8-016-induced reinstatement
Another two groups of rats underwent extinction, as described above, until drug-seeking behavior was extinguished. Afterwards, rats were tested for reinstatement of drug-seeking behavior triggered by R-MOD (0, 10, 30, and 100 mg/kg, i.p.) or JJC8-016 (0, 10, and 30 mg/kg, i.p.).
Experiment 5: Locomotor Activity
Locomotor effects by R-MOD or JJC8-016 alone
This experiment was designed to evaluate the psychomotor stimulating effects of R-MOD or JJC8-016 and also to see whether the reduction in cocaine-taking and cocaine-seeking behavior was because of nonspecific locomotor impairment or sedative effects. Drug-naive rats were placed in locomotor detection chambers (Accuscan, Columbus, OH) and habituated for 1 h. Each rat randomly received vehicle or one dose of R-MOD (0, 10, and 30 mg/kg, i.p.) or JJC8-016 (0, 10, and 30 mg/kg, i.p.). Following injection, locomotor activity was recorded for 2 h in 10 min intervals. Each animal was tested three times under different R-MOD or JJC8-016 doses in a counterbalanced manner. The time interval was 2–3 days between the tests. The distance counts (cm) were used to evaluate the effects of R-MOD or JJC8-016 on locomotion.
Effects of R-MOD or JJC8-016 on cocaine-enhanced locomotor activity
Another group of drug-naive rats were used to study the effect of R-MOD or JJC8-016 on cocaine-enhanced locomotor activity. After habituation to the locomotor detection chambers for 1 h, each rat randomly received vehicle or one dose of R-MOD (0, 10, and 30 mg/kg, i.p.) or JJC8-016 (0, 10, and 30 mg/kg, i.p.) and then received a single dose of cocaine (10 mg/kg, i.p.) 30 min later. Following the two injections, locomotor activity was recorded for 2 h in 10 min intervals. Each animal was tested three times under different R-MOD or JJC8-016 doses in a counterbalanced manner. The time interval between tests was 2–3 days.
Experiment 6: Electrical Brain-Stimulation Reward (BSR)
Surgery
Rats were anesthetized with sodium pentobarbital (65 mg/kg i.p.) and placed in a stereotaxic frame, and a monopolar stainless-steel stimulating electrode (Plastics One, Roanoke, VA) was placed unilaterally into the lateral hypothalamus using standard aseptic surgical and stereotaxic techniques. The implant coordinates for the tips of the electrodes were AP −2.56, ML ±1.9, and DV −8.6, according to the rat brain stereotaxic atlas (Paxinos and Watson, 1998). The electrode was attached to the skull with jeweler’s screws and dental acrylic. A wire leading from the electrode was wrapped around a skull screw to serve as a current return.
Initial training procedure
The experiments were conducted in standard Med Associates operant chambers (32 × 25 × 33 cm). Each operant chamber had a lever located 6.5 cm above the floor, connected to an electrical stimulator. The general procedures for electrical BSR were the same as we have reported previously (Hayes et al, 2003; Song et al, 2014; Vorel et al, 2002; Xi et al, 2006). Briefly, after 7 days of recovery from surgery, rats were allowed to self-train (auto shape) to lever press for rewarding BSR. Each press on the lever resulted in a 500 ms train of 0.1 ms rectangular cathodal pulses through the electrode in the rat’s lateral hypothalamus, followed by a 500 ms ‘timeout’ in which further presses did not produce brain stimulation. The initial stimulation parameters were 72 Hz and 200 mA. If the animal did not learn to lever-press, the stimulation intensity was increased daily by 50 mA until the animal learned to press (45–60 responses/30 s) or a maximum of 800 mA was reached. Rats that did not lever-press at 800 mA or in which the stimulation produced unwanted effects (eg, gross head or body movements, spinning, vocalization, or jumping) were removed from the experiment.
Rate-frequency training procedure
Following establishment of lever-pressing for BSR, rats were presented with a series of 16 different pulse frequencies, ranging from 141 to 25 Hz in descending order. At each pulse frequency, rats responded for two 30 s time periods (‘bins’), following which the pulse frequency was decreased by 0.05 log units. Following each 30 s bin, the lever retracted for 5 s. Throughout the experiments, rats were run for three sessions per day. Response rate for each frequency was defined as the mean number of lever responses during two 30 s bins. As lever-pressing behavior was variable during the first session (the ‘warm-up’ session), but was stable during the second and third sessions, the data from the first session were discarded, and the data from the second and third sessions were designated as the baseline session data and test session data, respectively. The BSR threshold (θ0) was defined as the minimum frequency at which the animal responded for rewarding stimulation.
Testing the effects of R-MOD or JJC8-016 on BSR
Once a baseline θ0 value was achieved (<15% variation over 5 continuous days), the effects of R-MOD and JJC8-016 on BSR were assessed. On test days, rats randomly received one of two different doses of R-MOD or JJC8-016 (10 and 30 mg/kg i.p.), or vehicle (10% DMSO and 15% Tween-80). After each test, rats received an additional 5–7 days of BSR restabilization until a new baseline θ0 was established. The order of testing for various doses of R-MOD and JJC8-016 was counterbalanced.
Experiment 7: In Vivo Brain Microdialysis
Microdialysis procedures used in the present experiments are the same as published before (Tanda et al, 2005). Microdialysis probes were prepared in the laboratory and had an active dialyzing surface of 1.8–2.0 mm (Tanda et al, 2005). Probes were surgically implanted into the NAc (according to the coordinates: anterior +2.0 mm, lateral ±1.0 mm from bregma, vertical −7.9 mm from dura) under a mixture of ketamine and xylazine anesthesia as described previously (Tanda et al, 2005).
Experiments were performed on freely moving rats, ∼22–24 h after the probe implantation. Artificial cerebrospinal fluid was perfused into the NAc and dialysates (10 μl) were sampled every 10 min and then immediately analyzed. Results are expressed as the percentage of the amount of DA in 10 min basal dialysate samples, calculated as means of DA values. After reaching stable DA values (2–4 consecutive samples, <10% variability), rats were treated with one dose of JJC8-016 (10 and 30 mg/kg, i.p.), and sampled every 10 min, for the first 2 h and every 20 min thereafter for a maximum of 3 h. Detection of DA in dialysate samples was accomplished by HPLC coupled with a coulometric detector (5200a Coulochem III, ESA, Chelmsford, MA), and cell potentials were set at +125 mV (oxidation) and −125 mV (reduction).
Data Analysis
All data are presented as means±SEM. One-way or two-way analysis of variance (ANOVA) for repeated measures over time or drug dose was used to analyze the effects of R-MOD or JJC8-016 in various experiments. Whenever a significant main effect was found, individual group comparisons were carried out using the Student–Newman–Keuls method.
Results
JJC8-016 Has Higher Affinity for DAT than R-MOD
Table 1 shows the chemical structures and previously reported in vitro binding affinities of R-MOD and JJC8-016 at DAT, SERT, the D2-like, and sigma1 receptors. At DAT, JJC8-016 (Ki=116 nM) had ∼26-fold higher affinity (lower Ki) than R-MOD (Ki=3050 nM). In addition, JJC8-016 also binds to SERT (Ki=360 nM) and the D2, D3, D4, and sigma1 receptors, whereas R-MOD was essentially inactive at these other receptors (Table 1). Indeed, across all the additional 68 receptor, transporter, and enzyme screen, at a concentration of 100 μM, R-MOD had no ‘hits.’ On the other hand, JJC8-016 had many ‘hits’ at a concentration of 10 μM (Supplementary Tables S1 and S2). Nevertheless, in addition to the binding profile recently described (Cao et al, 2016) JJC8-016 only showed Ca+2 channel binding at a concentration of 100 nM, in line with its DAT Ki value of 116 nM. Thus, these other off-target activities are likely not pharmacologically relevant.
JJC8-016 Inhibits Cocaine Self-Administration
The effects of systemic administration of R-MOD or JJC8-016 on cocaine self-administration demonstrated that R-MOD (10, 30, and 100 mg/kg) had no significant effect (Figure 1a, F3, 26=0.96, p>0.05), whereas JJC8-016 (10 and 30 mg/kg) significantly inhibited cocaine self-administration in a dose-dependent manner (Figure 1b, F2, 15=5.37, p<0.05). The post hoc individual group comparisons illustrated that JJC8-016, at 30 mg/kg, significantly decreased cocaine infusions (p<0.05). We also observed the effects of JJC8-016 on sucrose (a nondrug reinforcer) self-administration in rats. Figure 1c shows that JJC8-016 dose-dependently inhibited oral sucrose self-administration (F2, 18=6.82, p<0.05). In contrast, R-MOD, at the dose range of 30–100 mg/kg, failed to alter oral sucrose self-administration as we reported previously (Wang et al, 2015).
Effects of R-MOD and JJC8-016 on cocaine or sucrose self-administration. (a) R-MOD (10, 30, and 100 mg/kg) had no effect on cocaine self-administration. (b) JJC8-016 (10 and 30 mg/kg) dose-dependently inhibited cocaine self-administration. (c) JJC8-016, at the same doses, also inhibited oral sucrose self-administration. (d) R-MOD (10 and 30 mg/kg) shifted the cocaine dose–response curve downward and inhibited cocaine self-administration maintained by low doses (0.0313 and 0.0625 mg/kg/infusion) of cocaine. (e) JJC8-016 (10 and 30 mg/kg) dose-dependently shifted the cocaine dose–response curve downward and inhibited cocaine self-administration maintained by a wide dose range of cocaine (0.0313, 0.0625, 0.125, and 0.25 mg/kg/infusion). *P<0.05, **p<0.01, and ***p<0.001 compared with vehicle.
To further explore this finding, we observed the effects of R-MOD and JJC8-016 on the cocaine self-administration dose–response curve. We found that both R-MOD and JJC8-016 (10 and 30 mg/kg, i.p.) significantly shifted the cocaine dose–response curves downward, whereas JJC8-016 appeared to be more potent and effective than R-MOD (Figure 1d and e). Moreover, the inhibitory effects produced by R-MOD appeared to be cocaine dose dependent—R-MOD is more effective in cocaine self-administration maintained by very low doses of cocaine (0.03 and 0.06 mg/kg/infusion) (Figure 1d). Two-way ANOVA with repeated measures over cocaine and R-MOD dose revealed a significant treatment (vehicle vs R-MOD) main effect (F2, 105=2.87, p<0.05), significant cocaine dose main effect (F5, 105=26.94, p<0.001), and a significant treatment × dose interaction (F10, 105=2.24, p<0.05). Individual group comparisons revealed a significant reduction in cocaine self-administration after 10 mg/kg R-MOD treatment at cocaine doses of 0.03 (p<0.01) and 0.06 mg/kg/infusion cocaine (p<0.01) and after 30 mg/kg R-MOD treatment at cocaine doses of 0.06 mg/kg/infusion (p<0.01), when compared with the vehicle control group (Figure 1d).
Comparatively, at the same drug doses, JJC8-016 appeared to be more potent and effective than R-MOD in attenuating cocaine self-administration. JJC8-016 significantly inhibited cocaine self-administration maintained by multiple doses of cocaine (0.03, 0.06, 0.125, and 0.25 mg/kg/infusion) and significantly shifted the cocaine dose–response curve downward in a dose-dependent manner. Two-way ANOVA with two-factor repeated measures over cocaine and JJC8-016 dose revealed a significant treatment (vehicle vs JJC8-016) main effect (F2, 90=11.23, p<0.001), significant cocaine dose main effect (F5, 90=10.53, p<0.001), and a significant treatment × dose interaction (F10, 90=3.10, p<0.01). Individual group comparisons revealed a statistically significant reduction in cocaine self-administration after 10 mg/kg JJC8-016 treatment at cocaine doses of 0.03 mg/kg/infusion (p<0.01), 0.06 mg/kg/infusion (p<0.01), and 0.125 mg/kg/infusion (p<0.05) and after 30 mg/kg JJC8-016 treatment at cocaine dose of 0.03 mg/kg/infusion (p<0.001), 0.06 mg/kg/infusion (p<0.001), 0.125 mg/kg/infusion (p<0.001), and 0.25 mg/kg/infusion (p<0.01), when compared with the vehicle control group (Figure 1e).
JJC8-016 Attenuates Cocaine-Enhanced Locomotor Activity
To determine whether this inhibitory effect produced by R-MOD or JJC8-016 can be generalized to other actions of cocaine, we observed the effects of both DAT inhibitors on cocaine-enhanced locomotion. R-MOD had no significant effect on 10 mg/kg cocaine-enhanced locomotion (Figure 2a, F2, 357=0.30, p>0.05). However, JJC8-016 pretreatment significantly attenuated cocaine-enhanced locomotion in a dose-dependent manner (Figure 2b, F2, 357=2.62, p>0.05). Figure 2c and d show the locomotor effects of R-MOD and JJC8-016, respectively, without cocaine, illustrating that systemic administration of R-MOD dose-dependently increased locomotion (Figure 2c, F2, 357=10.09, p<0.001), whereas JJC8-016 had no effect (Figure 2d, F2, 357=0.65, p>0.05).
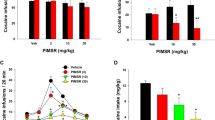
Effects of R-MOD and JJC8-016 on basal and cocaine-enhanced locomotor activity. (a) Pretreatment with R-MOD did not significantly alter cocaine-enhanced locomotor activity. (b) Pretreatment with JJC8-016 dose-dependently attenuated cocaine-induced increase in locomotion. (c) R-MOD alone dose-dependently increased locomotor activity. (d) JJC8-016 did not significantly alter basal level of locomotor behavior. Within-subjects design, n=8 in each treatment. *P<0.05, **p<0.01, and ***p<0.001 compared with vehicle control.
JJC8-016 Does Not Maintain Intravenous Self-Administration
We have recently reported that R-MOD cannot maintain i.v. self-administration by itself (Wang et al, 2015), suggesting that R-MOD is not rewarding as compared with cocaine. To determine whether JJC8-016 is rewarding and has similar addictive potential as cocaine, we carried out JJC8-016 self-administration studies in drug-naive rats in a manner similar to cocaine self-administration. Figure 3a shows that one group of naive rats quickly learned to lever press in order to receive cocaine infusions (0.5 mg/kg/infusion, i.v.), whereas another group of rats initially paired with JJC8-016 (1.0 mg/kg/infusion) failed to achieve self-administration behavior. Strikingly, when JJC8-016 was replaced by cocaine, rats displayed quick acquisition and stable self-administration behavior for cocaine. In contrast, when cocaine was replaced with JJC8-016 in another group of rats previously trained to self-administer cocaine, JJC8-016 substitution failed to maintain stable self-administration. Instead, gradual extinction behavior was observed. This pattern of extinction was essentially identical to saline substitution for cocaine, as we reported previously (Xi et al, 2006). Two-way ANOVA for repeated measures revealed a significant time main effect (F1, 154=20.56, p<0.001), drug main effect (F1, 14=121.27, p<0.001), and drug × time interaction (F1, 154=139.40, p<0.001). These findings suggest that JJC8-016 is not rewarding and is predicted to have low addictive liability.
Evaluation of the rewarding effects of R-modafinil and JJC8-016 in experimental animals. (a) Drug-naive rats rapidly acquired intravenous self-administration of cocaine, but not JJC8-016. JJC8-016 substitution for cocaine cannot maintain stable self-administration in rats previously experienced at self-administering cocaine, whereas cocaine substitution for JJC8-016 rapidly produced intravenous self-administration for cocaine in rats previously trained for JJC8-016 self-administration. (b) Systemic administration of JJC8-016, 10–30 mg/kg i.p., failed to alter extracellular dopamine (DA) in the nucleus accumbens. Results are means, with vertical bars representing SEM, of the amount of DA in 10 min dialysate samples, expressed as percentage of basal values. (c) Systemic administration of R-MOD dose-dependently decreased brain-stimulation reward (BSR) threshold (θ0), indicating enhanced brain reward. (d) Systemic administration of the same does of JJC8-016 had no effect on electrical BSR. *P<0.05, **p<0.01, and ***p<0.001 compared with the vehicle control group (c) or the basal levels of self-administration before the drug substitution test (a).
JJC8-016 Has No Effect on Extracellular DA in the Nucleus Accumbens
In an attempt to understand why JJC8-016 is not rewarding, we measured brain DA levels in the nucleus accumbens with in vivo microdialysis. Figure 3b shows that JJC8-016, at the same doses that significantly inhibited cocaine self-administration, had no significant effect on stimulation of extracellular DA in the nucleus accumbens (JJC8-016 treatment main effect: F1, 8=0.001, p>0.05, two-way ANOVA for repeated measures over time).
JJC8-016 Has No Effect on Electrical BSR
To further explore the above findings, we observed the effects of both R-MOD and JJC8-016 on electrical BSR. Systemic administration of R-MOD (10 and 30 mg/kg, i.p.) produced a significant reduction in brain-stimulation threshold (θ0 value) in a dose-dependent manner, suggesting an enhancement of BSR (Figure 3c, F2, 14=6.69, p<0.01). However, JJC8-016, at the same doses, had no effect on BSR (Figure 3d, F2, 14=0.35, p>0.05).
JJC8-016 Inhibits Cocaine-Induced Reinstatement of Drug-Seeking Behavior
We then explored the efficacy of R-MOD and JJC8-016 in preventing reinstatement to cocaine-seeking behavior. Figure 4 shows the total numbers of active lever presses observed during the last session of cocaine self-administration, the last session of extinction, and the reinstatement test session in two separate vehicle plus cocaine and different doses of R-MOD or JJC8-016 plus cocaine groups. A single noncontingent cocaine priming (10 mg/kg, i.p.) evoked robust reinstatement of cocaine-seeking behavior in rats extinguished from previous cocaine self-administration. Pretreatment with R-MOD (10 and 30 mg/kg) had no effect, whereas at the highest dose tested (100 mg/kg), R-MOD significantly attenuated cocaine-triggered reinstatement of drug-seeking behavior (Figure 4a, F3, 27=4.03, p<0.05). Individual group comparisons revealed a statistically significant reduction in cocaine-seeking behavior only after 100 mg/kg R-MOD (Figure 4a, t=4.49, p<0.05) administration. In contrast, pretreatment with JJC8-016 (10 and 30 mg/kg, i.p.) significantly attenuated cocaine-triggered reinstatement of drug-seeking behavior in a dose-dependent manner (Figure 4b, F2, 21=4.12, p<0.05). Individual group comparisons revealed a statistically significant reduction in cocaine-seeking behavior after 30 mg/kg JJC8-016 (p<0.05) administration when compared with the vehicle control group (Figure 4b). Figure 4c and d shows the effect produced by R-MOD or JJC8-016 alone in reinstatement testing without cocaine, illustrating that, at the same doses (10, 30 mg/kg), neither R-MOD nor JJC8-016 alone produced reinstatement. However, at the high dose (100 mg/kg), R-MOD alone induced reinstatement of drug-seeking behavior (Figure 4c, F3, 23=5.29, p<0.01; Figure 4d, F2, 19=0.59, p>0.05).
Effects of R-MOD and JJC8-016 on cocaine-primed reinstatement of cocaine-seeking behavior. (a, b) Active lever responses during the last session of cocaine self-administration, last session of extinction, and reinstatement testing illustrating that pretreatment with R-MOD (a) or JJC8-016 (b) dose-dependently inhibited cocaine-triggered reinstatement of drug-seeking behavior. A threefold higher dose (100 mg/kg) of R-MOD than JJC8-016 (30 mg/kg) is required to produce this inhibitory effect. (c, d) Active lever responses during the last session of cocaine self-administration, extinction, and reinstatement testing illustrating that R-MOD, at 100 mg/kg, induced reinstatement of cocaine-seeking behavior, whereas JJC8-016 did not at each drug dose (10 and 30 mg/kg). *P<0.05 and **p<0.001 compared with the vehicle control.
Discussion
The major findings in this study include the following. (1) JJC8-016 is a novel DAT ligand with higher affinity for DAT than R-MOD, but has no effect on extracellular DA, suggesting that JJC8-016 binding to the DAT may not significantly alter DAT (reuptake) in vivo, at the doses tested, although complete inhibition of [3H]DA uptake in a cell-based assay has been described (Cao et al, 2016). This is significantly different from the typical DAT inhibitors, including cocaine. In addition, JJC8-016 also has significant binding affinities for SERT, D2-like DA, and sigma1 receptors, whereas R-MOD is DAT selective, although with significantly lower DAT affinity than JJC8-016. (2) Systemic administration of JJC8-016 significantly and dose-dependently inhibits intravenous cocaine self-administration, cocaine-enhanced locomotion, and cocaine-induced reinstatement of drug-seeking behavior, whereas R-MOD inhibits cocaine-induced reinstatement only at the highest dose (100 mg/kg) tested. (3) R-MOD itself displays rewarding and psychomotor-stimulant effects as assessed by enhanced locomotor activities, enhanced brain-stimulation reward, and reinstatement of drug-seeking behavior, whereas JJC8-016 does not, as assessed by the lack of effects in these behavioral assays, in neurochemical (microdialysis) assays, and in the drug substitution test in self-administration experiments. Taken together, these findings suggest that JJC8-016 has attributes that look promising for development as a pharmacotherapeutic treatment of cocaine use disorders, as it is more potent and effective than R-MOD in attenuating cocaine’s actions and is predicted to have no significant abuse potential.
Cocaine produces rewarding and psychomotor-stimulating effects mainly through the inhibition of DA uptake via the DAT. Accordingly, it has been proposed that other DAT inhibitors may possess similar addictive liability as cocaine (Kuhar et al, 1991; Ritz et al, 1987). In contrast, several classes of small molecules have been reported to have high affinity and selective DAT binding profiles, but without the behavioral profile of cocaine that is associated with its reinforcing effects and abuse liability (Cao et al, 2010; Desai et al, 2005; Hiranita et al, 2009; Hong et al, 2016; Katz et al, 2004; Loland et al, 2012; Newman et al, 1994, 1995; Tanda et al, 2009). These ligands have been coined atypical DAT blockers or inhibitors (Reith et al, 2015). The atypical DAT inhibitors, such as tropane-based benztropine-like analogs (eg, JHW 007) have structural similarities to cocaine, but have very different structure–activity relationships (Agoston et al, 1997; Newman et al, 1994, 1995; Zou et al, 2003), suggesting a distinct binding mode at the DAT. Indeed, these analogs appear to bind differently than cocaine and cocaine-like molecules at the DAT that has been proposed to affect their behavioral profiles in vivo (Beuming et al, 2008; Loland et al, 2008). These behaviors include reduced or complete lack of cocaine-like rewarding effects (Desai et al, 2005; Hiranita et al, 2009; Tanda et al, 2009). These studies collectively demonstrate that not all DAT inhibitors have abuse liability and some may be effective in attenuating cocaine-induced reward. As such, we posit that these atypical DAT inhibitors may have potential as medication candidates to treat cocaine dependence.
As discussed previously, (±)MOD is another clinically available and unique DAT inhibitor that is a mild psychostimulant-like agent that increases wakefulness, improves attention, and enhances cognitive performance (Marchant et al, 2009; Tsanov et al, 2010; Turner et al, 2004). However, it does not serve as a reinforcer in cocaine abusers (Vosburg et al, 2010). As such, (±)MOD has been evaluated clinically as a candidate drug for the treatment of cocaine addiction. For example, (±)MOD was reported to attenuate craving during cocaine withdrawal and decrease smoked cocaine self-administration in humans (Ballon and Feifel, 2006; Dackis et al, 2005; Hart et al, 2008). However, other studies suggest that (±)MOD is not effective in attenuating impulsivity for cocaine-taking and attentional bias in crack-cocaine-dependent patients (Nuijten et al, 2016). In general, (±)MOD has been reported to have low abuse liability (Mereu et al, 2013), but a rare case of dependence was recently reported when a high dose was given (Krishnan and Chary, 2015). In total, these reports suggest that (±)MOD may not be ideal for the treatment of cocaine dependence in humans. As R-MOD is also clinically available, it has been suggested as an alternative to (±)MOD for evaluation in cocaine-dependent subjects (Loland et al, 2012; Mereu et al, 2013). Nevertheless, in contrast to our findings in nicotine self-administering rats, R-MOD was ineffective in attenuating cocaine self-administration. This may be because of its much lower affinity for DAT (Ki=3050 nM) than cocaine (Ki=72 nM) (Cao et al, 2010) that may prevent it from effectively blocking cocaine from binding to the DAT. This inspired us to modify the MOD chemical structure in order to develop novel analogs with higher affinities as DAT inhibitors than MOD itself (Cao et al, 2010; Okunola-Bakare et al, 2014). JJC8-016 was identified as a lead compound and has 26-fold higher affinity for DAT than R-MOD. In contrast to R-MOD, JJC8-016 also has higher affinities for SERT, sigma1 receptor, and D2-like (D2, D3, D4) receptors (Table 1).
One of the most important findings in the present study is that JJC8-016 appears to be more potent and effective than R-MOD in attenuating cocaine-taking and cocaine-seeking behavior. This may be related to its higher binding affinity of JJC8-016 for the DAT than R-MOD. JJC8-016 would be predicted to interfere with cocaine binding to the DAT and therefore attenuate cocaine reward and relapse to drug-seeking behavior. Furthermore, JJC8-016 also displayed high affinity for D2-like (particularly D3 and D4) DA receptors and sigma1 receptors. We and others have previously reported that D3 receptor antagonists and DAT/sigma1 receptor dual inhibitors are highly effective in attenuating cocaine self-administration (Bari and Pierce, 2005; Cao et al, 2003; Hiranita et al, 2011; Katz et al, 2016a, b; Maurice et al, 2002; Song et al, 2014). These findings suggest that these non-DAT mechanisms may also contribute to the behavioral effects produced by JJC8-016. Clearly, more studies are required to further address the functional role of the off-target interactions in mediating the therapeutic effects of JJC8-016. Furthermore, JJC8-016 also attenuates sucrose self-administration, whereas other atypical DAT inhibitors (R-MOD, JHW 007) do not (Ferragud et al, 2014; Wang et al, 2015). This inhibitory effect may be related to the binding of JJC8-016 to other off targets. In addition, more studies with chronic drug doses are warranted to determine whether these are nonspecific actions or whether the attenuation of cocaine-seeking and cocaine-taking behavior would persist, whereas effects on sucrose intake would not.
Another important finding in the present study is that JJC8-016 has no or low abuse potential. This is supported by the findings that naive rats self-administered cocaine, but not JJC8-016. JJC8-016 substitution also failed to maintain self-administration behavior in rats previously trained to self-administer cocaine. JJC8-016 also failed to elevate extracellular dopamine and basal levels of locomotion or alter electrical BSR behavior. It also failed to evoke reinstatement of drug-seeking behavior in rats after drug-seeking behavior is extinguished. As JJC8-016 (Ki=116 nM) displayed similarly high affinity for DAT as cocaine (Ki=72 nM), these data support an atypical DAT inhibitor profile for JJC8-016.
Considerable evidence suggests that the binding mode of DA uptake inhibitors at the DAT can affect downstream behavioral actions (Loland et al, 2008, 2012; Schmitt and Reith, 2011), although not all atypical DAT inhibitors bind to the DAT in a similar manner (Hong et al, 2016). This notion has been further supported by site-directed mutagenesis data, in which JJC8-016, like the benztropine-like atypical DAT inhibitors (eg, JHW 007), does not prefer binding to the outward open conformation of the DAT, as cocaine does (Cao et al, 2016). Moreover, JJC8-016 also binds to SERT and several D2-like receptors (D2, D3, and D4) as well as the sigma1 receptor. We have determined that JJC8-016 is a weak (low potency) D2 antagonist (IC50 >10 μM) (Cao et al, 2016) in an in vitro functional assay. As we did not observe any behaviors that are typically associated with D2 antagonism, it is unlikely that this mechanism is involved in the behaviors observed. Furthermore, although the mechanistic role of these non-DAT targets in the behavioral profile of JJC8-016 are unclear, there is no evidence to support that actions at these sites would be rewarding and cause JJC8-016 to be addictive.
A third important finding is that R-MOD is not effective in attenuating cocaine’s actions in multiple animal models of cocaine abuse. The simplest explanation is that R-MOD has much lower affinity than cocaine for DAT and thus at the doses achievable, because of its limited solubility, R-MOD cannot prevent cocaine from binding sufficiently to reduce its pharmacological effects. Therefore, much higher doses of R-MOD may be required to block cocaine’s binding to the DAT, at least in rats. This is clearly supported by our findings that R-MOD, at the same doses of JJC8-016 that inhibited cocaine self-administration, did inhibit cocaine self-administration maintained by very low doses of cocaine in the multiple-dose cocaine self-administration experiment and that a threefold higher dose (100 mg/kg) of R-MOD inhibited cocaine-induced reinstatement of drug-seeking behavior. In contrast to JJC8-016, R-MOD exhibits rewarding and psychomotor-stimulating effects by itself as assessed by an increase in electrical BSR, extracellular DA in the NAc and open-field locomotion, as well as in reinstatement produced by R-MOD alone. This is likely related to the DA-elevating effects of R-MOD (Loland et al, 2012). Indeed, although there are few reports of abuse liability of R-MOD (Jerry et al, 2016), its mild psychostimulant effects are likely clinically relevant to its effectiveness for sleep disorders, for which it is clinically used. As (±)MOD has recently been reported to be effective in a subpopulation of cocaine-dependent subjects (Kampman et al, 2015), R-MOD might also be effective. Hence, clinical investigation of this drug may be warranted, despite the lack of efficacy in the rodent models reported herein.
Finally, the reduction in cocaine self-administration and reinstatement of drug-seeking behavior is not because of sedation or locomotor impairment after JJC8-016 administration, as JJC8-016 had no significant effect on locomotor activity. In addition, JJC8-016 also failed to alter active lever responses for electrical BSR and inactive lever response during the cocaine self-administration and reinstatement tests, suggesting that the rats are not impaired in the presence of behaviorally effective doses of this drug.
In conclusion, the present study demonstrates that JJC8-016 is an atypical DAT inhibitor that has moderately high affinity for the DAT, has no effect on extracellular DA in the nucleus accumbens, in vivo, despite its potent inhibition of [3H]DA uptake in a cell-based assay (Cao et al, 2016), and has no addictive potential. In addition, JJC8-016 has significant off-target activity at the dopamine D3 and D4 receptor subtypes, as well as the sigma1 receptor and SERT. Indeed, it is likely that some or all of these off-target actions contribute to its unique behavioral profile and medication development potential. Strikingly, it is more potent and effective than R-MOD in attenuating cocaine self-administration and reinstatement of drug-seeking behavior and thus serves as a lead to new and promising pharmacotherapies for the treatment of cocaine use disorders.
Funding and disclosure
This research was supported by the Intramural Research Program of the National Institute on Drug Abuse (Z1A DA000389), National Institutes of Health, USA. The authors declare no conflict of interest.
References
Agoston GE, Wu JH, Izenwasser S, George C, Katz J, Kline RH et al (1997). Novel N-substituted 3 alpha-[bis(4'-fluorophenyl)methoxy]tropane analogues: selective ligands for the dopamine transporter. J Med Chem 40: 4329–4339.
Anderson AL, Li SH, Biswas K, McSherry F, Holmes T, Iturriaga E et al (2012). Modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend 120: 135–141.
Ballon JS, Feifel D (2006). A systematic review of modafinil: potential clinical uses and mechanisms of action. J Clin Psychiatry 67: 554–566.
Bari AA, Pierce RC (2005). D1-like and D2 dopamine receptor antagonists administered into the shell subregion of the rat nucleus accumbens decrease cocaine, but not food, reinforcement. Neuroscience 135: 959–968.
Beuming T, Kniazeff J, Bergmann ML, Shi L, Gracia L, Raniszewska K et al (2008). The binding sites for cocaine and dopamine in the dopamine transporter overlap. Nat Neurosci 11: 780–789.
Camacho A, Stein MB (2002). Modafinil for social phobia and amphetamine dependence. Am J Psychiatry 159: 1947–1948.
Cao J, Kulkarni SS, Husbands SM, Bowen WD, Williams W, Kopajtic T et al (2003). Dual probes for the dopamine transporter and sigma1 receptors: novel piperazinyl alkyl-bis(4'-fluorophenyl)amine analogues as potential cocaine-abuse therapeutic agents. J Med Chem 46: 2589–2598.
Cao J, Prisinzano TE, Okunola OM, Kopajtic T, Shook M, Katz JL et al (2010). Structure-activity relationships at the monoamine transporters for a novel series of modafinil (2-[(diphenylmethyl)sulfinyl]acetamide) analogues. ACS Med Chem Lett 2: 48–52.
Cao J, Slack RD, Bakare OM, Burzynski C, Rais R, Slusher BS et al (2016). Novel and high affinity 2-[(diphenylmethyl)sulfinyl]acetamide (modafinil) analogues as atypical dopamine transporter inhibitors. J Med Chem 59: 10676–10691.
Czoty PW, Stoops WW, Rush CR (2016). Evaluation of the ‘pipeline’ for development of medications for cocaine use disorder: a review of translational preclinical, human laboratory, and clinical trial research. Pharmacol Rev 68: 533–562.
Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O'Brien CP (2005). A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology 30: 205–211.
Dackis CA, Kampman KM, Lynch KG, Plebani JG, Pettinati HM, Sparkman T et al (2012). A double-blind, placebo-controlled trial of modafinil for cocaine dependence. J Subst Abuse Treat 43: 303–312.
Dackis CA, Lynch KG, Yu E, Samaha FF, Kampman KM, Cornish JW et al (2003). Modafinil and cocaine: a double-blind, placebo-controlled drug interaction study. Drug Alcohol Depend 70: 29–37.
De La Garza R 2nd, Zorick T, London ED, Newton TF (2010). Evaluation of modafinil effects on cardiovascular, subjective, and reinforcing effects of methamphetamine in methamphetamine-dependent volunteers. Drug Alcohol Depend 106: 173–180.
Desai RI, Kopajtic TA, Koffarnus M, Newman AH, Katz JL (2005). Identification of a dopamine transporter ligand that blocks the stimulant effects of cocaine. J Neurosci 25: 1889–1893.
Di Chiara G, Imperato A (1988). Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85: 5274–5278.
Ferragud A, Velazquez-Sanchez C, Canales JJ (2014). Modulation of methamphetamine's locomotor stimulation and self-administration by JHW 007, an atypical dopamine reuptake blocker. Eur J Pharmacol 731: 73–79.
Garnock-Jones KP, Dhillon S, Scott LJ (2009). Armodafinil. CNS Drugs 23: 793–803.
Goudriaan AE, Veltman DJ, van den Brink W, Dom G, Schmaal L (2013). Neurophysiological effects of modafinil on cue-exposure in cocaine dependence: a randomized placebo-controlled cross-over study using pharmacological fMRI. Addict Behav 38: 1509–1517.
Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW (2008). Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology 33: 761–768.
Hayes RJ, Vorel SR, Spector J, Liu X, Gardner EL (2003). Electrical and chemical stimulation of the basolateral complex of the amygdala reinstates cocaine-seeking behavior in the rat. Psychopharmacology 168: 75–83.
Heinzerling KG, Swanson AN, Kim S, Cederblom L, Moe A, Ling W et al (2010). Randomized, double-blind, placebo-controlled trial of modafinil for the treatment of methamphetamine dependence. Drug Alcohol Depend 109: 20–29.
Hiranita T, Soto PL, Kohut SJ, Kopajtic T, Cao J, Newman AH et al (2011). Decreases in cocaine self-administration with dual inhibition of the dopamine transporter and sigma receptors. J Pharmacol Exp Thers 339: 662–677.
Hiranita T, Soto PL, Newman AH, Katz JL (2009). Assessment of reinforcing effects of benztropine analogs and their effects on cocaine self-administration in rats: comparisons with monoamine uptake inhibitors. J Pharmacol Exp Ther 329: 677–686.
Hong WC, Kopajtic TA, Xu L, Lomenzo SA, Jean B, Madura JD et al (2016). 2-Substituted 3beta-aryltropane cocaine analogs produce atypical effects without inducing inward-facing dopamine transporter conformations. J Pharmacol Exp Ther 356: 624–634.
Howell LL, Wilcox KM (2001). The dopamine transporter and cocaine medication development: drug self-administration in nonhuman primates. J Pharmacol Exp Ther 298: 1–6.
Jerry JM, Shirvani N, Dale R (2016). Addiction to armodafinil and modafinil presenting with paranoia. J Clin Psychopharmacol 36: 98–100.
Kampman KM, Lynch KG, Pettinati HM, Spratt K, Wierzbicki MR, Dackis C et al (2015). A double blind, placebo controlled trial of modafinil for the treatment of cocaine dependence without co-morbid alcohol dependence. Drug Alcohol Depend 155: 105–110.
Katz JL, Hiranita T, Kopajtic TA, Rice KC, Mesangeau C, Narayanan S et al (2016a). Blockade of cocaine or sigma receptor agonist self administration by subtype-selective sigma receptor antagonists. J Pharmacol Exp Ther 358: 109–124.
Katz JL, Hong WC, Hiranita T, Su TP (2016b). A role for sigma receptors in stimulant self-administration and addiction. Behav Pharmacol 27 (2-3 Spec Issue): 100–115.
Katz JL, Kopajtic TA, Agoston GE, Newman AH (2004). Effects of N-substituted analogs of benztropine: diminished cocaine-like effects in dopamine transporter ligands. J Pharmacol Exp Ther 309: 650–660.
Krishnan R, Chary KV (2015). A rare case modafinil dependence. J Pharmacol Pharmacother 6: 49–50.
Kuhar MJ, Ritz MC, Boja JW (1991). The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci 14: 299–302.
Le AD, Li Z, Funk D, Shram M, Li TK, Shaham Y (2006). Increased vulnerability to nicotine self-administration and relapse in alcohol-naive offspring of rats selectively bred for high alcohol intake. J Neurosci 26: 1872–1879.
Loland CJ, Desai RI, Zou MF, Cao J, Grundt P, Gerstbrein K et al (2008). Relationship between conformational changes in the dopamine transporter and cocaine-like subjective effects of uptake inhibitors. Mol Pharmacol 73: 813–823.
Loland CJ, Mereu M, Okunola OM, Cao J, Prisinzano TE, Mazier S et al (2012). R-modafinil (armodafinil): a unique dopamine uptake inhibitor and potential medication for psychostimulantabuse. Biol Psychiatry 72: 405–413.
Luscher C, Malenka RC (2011). Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69: 650–663.
Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L et al (2006). Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther 319: 561–569.
Mahler SV, Hensley-Simon M, Tahsili-Fahadan P, LaLumiere RT, Thomas C, Fallon RV et al (2014). Modafinil attenuates reinstatement of cocaine seeking: role for cystine-glutamate exchange and metabotropic glutamate receptors. Addict Biol 19: 49–60.
Malcolm R, Swayngim K, Donovan JL, DeVane CL, Elkashef A, Chiang N et al (2006). Modafinil and cocaine interactions. Am J Drug Alcohol Abuse 32: 577–587.
Mantsch JR, Li SJ, Risinger R, Awad S, Katz E, Baker DA et al (2007). Levo-tetrahydropalmatine attenuates cocaine self-administration and cocaine-induced reinstatement in rats. Psychopharmacology 192: 581–591.
Marchant NL, Kamel F, Echlin K, Grice J, Lewis M, Rusted JM (2009). Modafinil improves rapid shifts of attention. Psychopharmacology 202: 487–495.
Maurice T, Martin-Fardon R, Romieu P, Matsumoto RR (2002). Sigma(1) (sigma(1)) receptor antagonists represent a new strategy against cocaine addiction and toxicity. Neurosci Biobehav Rev 26: 499–527.
McGregor C, Srisurapanont M, Mitchell A, Wickes W, White JM (2008). Symptoms and sleep patterns during inpatient treatment of methamphetamine withdrawal: a comparison of mirtazapine and modafinil with treatment as usual. J Subst Abuse Treat 35: 334–342.
Mereu M, Bonci A, Newman AH, Tanda G (2013). The neurobiology of modafinil as an enhancer of cognitive performance and a potential treatment for substance use disorders. Psychopharmacology 229: 415–434.
Mereu M, Chun LE, Prisinzano TE, Newman AH, Katz JL, Tanda G (2017). The unique psychostimulant profile of (+/−)-modafinil: investigation of behavioral and neurochemical effects in mice. Eur J Neurosci 45: 167–174.
Mignot E, Nishino S, Guilleminault C, Dement WC (1994). Modafinil binds to the dopamine uptake carrier site with low affinity. Sleep 17: 436–437.
Minzenberg MJ, Carter CS (2008). Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology 33: 1477–1502.
Newman AH, Allen AC, Izenwasser S, Katz JL (1994). Novel 3 alpha-(diphenylmethoxy)tropane analogs: potent dopamine uptake inhibitors without cocaine-like behavioral profiles. J Med Chem 37: 2258–2261.
Newman AH, Kline RH, Allen AC, Izenwasser S, George C, Katz JL (1995). Novel 4'-substituted and 4',4’-disubstituted 3 alpha-(diphenylmethoxy)tropane analogs as potent and selective dopamine uptake inhibitors. J Med Chem 38: 3933–3940.
Newman AH, Kulkarni S (2002). Probes for the dopamine transporter: new leads toward a cocaine-abuse therapeutic—A focus on analogues of benztropine and rimcazole. Med Res Rev 22: 429–464.
Nuijten M, Blanken P, Van den Brink W, Goudriaan AE, Hendriks VM (2016). Impulsivity and attentional bias as predictors of modafinil treatment outcome for retention and drug use in crack-cocaine dependent patients: Results of a randomised controlled trial. J Psychopharmacol 30: 616–626.
Okunola-Bakare OM, Cao J, Kopajtic T, Katz JL, Loland CJ, Shi L et al (2014). Elucidation of structural elements for selectivity across monoamine transporters: novel 2-[(diphenylmethyl)sulfinyl]acetamide (modafinil) analogues. J Med Chem 57: 1000–1013.
Paxinos G, Watson C (1998) The Rat Brain in Stereotaxic Coordinates. Academic Press.
Platt DM, Rowlett JK, Spealman RD (2002). Behavioral effects of cocaine and dopaminergic strategies for preclinical medication development. Psychopharmacology (Berl) 163: 265–282.
Reichel CM, See RE (2010). Modafinil effects on reinstatement of methamphetamine seeking in a rat model of relapse. Psychopharmacology 210: 337–346.
Reichel CM, See RE (2012). Chronic modafinil effects on drug-seeking following methamphetamine self-administration in rats. Int J Neuropsychopharmacol 15: 919–929.
Reith ME, Blough BE, Hong WC, Jones KT, Schmitt KC, Baumann MH et al (2015). Behavioral, biological, and chemical perspectives on atypical agents targeting the dopamine transporter. Drug Alcohol Depend 147: 1–19.
Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ (1987). Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237: 1219–1223.
Robertson P Jr, Hellriegel ET (2003). Clinical pharmacokinetic profile of modafinil. Clin Pharmacokinet 42: 123–137.
Rothman RB, Baumann MH, Prisinzano TE, Newman AH (2008). Dopamine transport inhibitors based on GBR12909 and benztropine as potential medications to treat cocaine addiction. Biochem Pharmacol 75: 2–16.
Rothman RB, Glowa JR (1995). A review of the effects of dopaminergic agents on humans, animals, and drug-seeking behavior, and its implications for medication development. Focus on GBR 12909. Mol Neurobiol 11: 1–19.
Runyon SP, Carroll FI (2006). Dopamine transporter ligands: recent developments and therapeutic potential. Curr Top Med Chem 6: 1825–1843.
Schmaal L, Goudriaan AE, Joos L, Kruse AM, Dom G, van den Brink W et al (2013). Modafinil modulates resting-state functional network connectivity and cognitive control in alcohol-dependent patients. Biol Psychiatry 73: 789–795.
Schmitt KC, Reith ME (2011). The atypical stimulant and nootropic modafinil interacts with the dopamine transporter in a different manner than classical cocaine-like inhibitors. PLoS ONE 6: e25790.
Shearer J, Darke S, Rodgers C, Slade T, van Beek I, Lewis J et al (2009). A double-blind, placebo-controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction 104: 224–233.
Song R, Bi GH, Zhang HY, Yang RF, Gardner EL, Li J et al (2014). Blockade of D3 receptors by YQA14 inhibits cocaine's rewarding effects and relapse to drug-seeking behavior in rats. Neuropharmacology 77: 398–405.
Song R, Yang RF, Wu N, Su RB, Li J, Peng XQ et al (2012). YQA14: a novel dopamine D3 receptor antagonist that inhibits cocaine self-administration in rats and mice, but not in D3 receptor-knockout mice. Addict Biol 17: 259–273.
Swanson LW (1982). The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull 9: 321–353.
Tahsili-Fahadan P, Carr GV, Harris GC, Aston-Jones G (2010). Modafinil blocks reinstatement of extinguished opiate-seeking in rats: mediation by a glutamate mechanism. Neuropsychopharmacology 35: 2203–2210.
Tanda G, Ebbs A, Newman AH, Katz JL (2005). Effects of 4'-chloro-3 alpha-(diphenylmethoxy)-tropane on mesostriatal, mesocortical, and mesolimbic dopamine transmission: comparison with effects of cocaine. J Pharmacol Exp Ther 313: 613–620.
Tanda G, Newman AH, Katz JL (2009). Discovery of drugs to treat cocaine dependence: behavioral and neurochemical effects of atypical dopamine transport inhibitors. Adv Pharmacol 57: 253–289.
Torregrossa MM, Kalivas PW (2008). Microdialysis and the neurochemistry of addiction. Pharmacol Biochem Behav 90: 261–272.
Tsanov M, Lyons DG, Barlow S, Gonzalez Reyes RE, O'Mara SM (2010). The psychostimulant modafinil facilitates water maze performance and augments synaptic potentiation in dentate gyrus. Neuropharmacology 59: 9–19.
Turner DC, Clark L, Dowson J, Robbins TW, Sahakian BJ (2004). Modafinil improves cognition and response inhibition in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 55: 1031–1040.
Verrico CD, Haile CN, Mahoney JJ 3rd, Thompson-Lake DG, Newton TF, De La Garza R 2nd (2014). Treatment with modafinil and escitalopram, alone and in combination, on cocaine-induced effects: a randomized, double blind, placebo-controlled human laboratory study. Drug Alcohol Depend 141: 72–78.
Vorel SR, Ashby CR Jr, Paul M, Liu X, Hayes R, Hagan JJ et al (2002). Dopamine D3 receptor antagonism inhibits cocaine-seeking and cocaine-enhanced brain reward in rats. J Neurosci 22: 9595–9603.
Vosburg SK, Hart CL, Haney M, Rubin E, Foltin RW (2010). Modafinil does not serve as a reinforcer in cocaine abusers. Drug Alcohol Depend 106: 233–236.
Wang XF, Bi GH, He Y, Yang HJ, Gao JT, Okunola-Bakare OM et al (2015). R-modafinil attenuates nicotine-taking and nicotine-seeking behavior in alcohol-preferring rats. Neuropsychopharmacology 40: 1762–1771.
Wise RA, Mendrek A, Carlezon WA Jr (1996). MK-801 (dizocilpine): synergist and conditioned stimulus in bromocriptine-induced psychomotor sensitization. Synapse 22: 362–368.
Wong YN, King SP, Simcoe D, Gorman S, Laughton W, McCormick GC et al (1999). Open-label, single-dose pharmacokinetic study of modafinil tablets: influence of age and gender in normal subjects. J Clin Pharmacol 39: 281–288.
Xi ZX, Gilbert JG, Pak AC, Ashby CR Jr, Heidbreder CA, Gardner EL (2005). Selective dopamine D3 receptor antagonism by SB-277011A attenuates cocaine reinforcement as assessed by progressive-ratio and variable-cost-variable-payoff fixed-ratio cocaine self-administration in rats. Eur J Neurosci 21: 3427–3438.
Xi ZX, Newman AH, Gilbert JG, Pak AC, Peng XQ, Ashby CR Jr et al (2006). The novel dopamine D3 receptor antagonist NGB 2904 inhibits cocaine's rewarding effects and cocaine-induced reinstatement of drug-seeking behavior in rats. Neuropsychopharmacology 31: 1393–1405.
Xi ZX, Song R, Li X, Lu GY, Peng XQ, He Y et al (2016). Compound 32, 476: a promising agonist therapy for cocaine addiction. Neuropsychopharmacology 42: 682–694.
Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V et al (2009). Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther 329: 738–746.
Zou MF, Kopajtic T, Katz JL, Newman AH (2003). Structure-activity relationship comparison of (S)-2beta-substituted 3alpha-(bis[4-fluorophenyl]methoxy)tropanes and (R)-2beta-substituted 3beta-(3,4-dichlorophenyl)tropanes at the dopamine transporter. J Med Chem 46: 2908–2916.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, HY., Bi, GH., Yang, HJ. et al. The Novel Modafinil Analog, JJC8-016, as a Potential Cocaine Abuse Pharmacotherapeutic. Neuropsychopharmacol 42, 1871–1883 (2017). https://doi.org/10.1038/npp.2017.41
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2017.41
This article is cited by
-
cAMP-mediated upregulation of HCN channels in VTA dopamine neurons promotes cocaine reinforcement
Molecular Psychiatry (2023)
-
Interactions of calmodulin kinase II with the dopamine transporter facilitate cocaine-induced enhancement of evoked dopamine release
Translational Psychiatry (2023)
-
Reinstatement of synaptic plasticity in the aging brain through specific dopamine transporter inhibition
Molecular Psychiatry (2021)
-
Improving translation of animal models of addiction and relapse by reverse translation
Nature Reviews Neuroscience (2020)
-
Translating the atypical dopamine uptake inhibitor hypothesis toward therapeutics for treatment of psychostimulant use disorders
Neuropsychopharmacology (2019)