Abstract
Cocaine use disorder is a global public health problem for which there are no Food and Drug Administration-approved pharmacotherapies. Emerging preclinical evidence has implicated both serotonin (5-HT) 2C and 2A receptors as potential mechanisms for mediating serotonergic attenuation of cocaine abuse-related neurochemical and behavioral effects. Therefore, the present study aim was to determine whether repeated 7-day treatment with the 5-HT2C agonist lorcaserin (0.1–1.0 mg/kg per day, intramuscular; 0.032–0.1 mg/kg/h, intravenous) or the 5-HT2A inverse agonist/antagonist pimavanserin (0.32–10 mg/kg per day, intramuscular) attenuated cocaine reinforcement under a concurrent ‘choice’ schedule of cocaine and food availability in rhesus monkeys. During saline treatment, cocaine maintained a dose-dependent increase in cocaine vs food choice. Repeated pimavanserin (3.2 mg/kg per day) treatments significantly increased small unit cocaine dose choice. Larger lorcaserin (1.0 mg/kg per day and 0.1 mg/kg/h) and pimavanserin (10 mg/kg per day) doses primarily decreased rates of operant behavior. Coadministration of ineffective lorcaserin (0.1 mg/kg per day) and pimavanserin (0.32 mg/kg per day) doses also failed to significantly alter cocaine choice. These results suggest that neither 5-HT2C receptor activation nor 5-HT2A receptor blockade are sufficient to produce a therapeutic-like decrease in cocaine choice and a complementary increase in food choice. Overall, these results do not support the clinical utility of 5-HT2C agonists and 5-HT2A inverse agonists/antagonists alone or in combination as candidate anti-cocaine use disorder pharmacotherapies.
Similar content being viewed by others
Introduction
Cocaine use disorder is a worldwide public health problem for which there are no approved pharmacotherapies (Acri and Skolnick, 2013; Czoty et al, 2016) Serotonin (5-HT) 5-HT2C and 5-HT2A receptors modulate mesolimbic dopamine neurotransmission that mediates the abuse-related effects of cocaine, and as a result, these receptors have emerged as pharmacological targets for candidate cocaine use disorder medications (Alex and Pehek, 2007; Howell and Cunningham, 2015). Both 5-HT2C and 5-HT2A receptors are excitatory Gq/G11-protein coupled receptors (Hannon and Hoyer, 2008), but their distinct anatomical locations within the mesolimbic dopamine system result in distinct effects on dopaminergic neurotransmission. 5-HT2C receptors are located primarily on GABA-ergic neurons that inhibit dopamine neurons, and as a result, stimulation of 5-HT2C receptors excites GABA neurons, subsequently inhibits dopamine neurons, and attenuates abuse-related effects of cocaine. For example, acute pretreatment with the 5-HT2C agonist RO 60-0175 attenuated cocaine-induced extracellular nucleus accumbens (NAc) dopamine increases in both rats (Cathala et al, 2015; Navailles et al, 2008) and monkeys (Manvich et al, 2012). Consistent with this neurochemical evidence, acute pretreatments with RO 60-0175 or the 5-HT2C agonist lorcaserin in rats and monkeys attenuated cocaine discrimination (Callahan and Cunningham, 1995; Collins et al, 2016), cocaine self-administration (Collins et al, 2016; Cunningham et al, 2011; Fletcher et al, 2008; Gerak et al, 2016; Grottick et al, 2000; Harvey-Lewis et al, 2016; Manvich et al, 2012) and cocaine-induced reinstatement of extinguished cocaine self-administration (Cunningham et al, 2011; Gerak et al, 2016; Harvey-Lewis et al, 2016; Manvich et al, 2012; Rüedi-Bettschen et al, 2015). Additionally, lorcaserin-induced decreases in cocaine self-administration were sustained during chronic lorcaserin treatment in monkeys (Collins et al, 2016; Gerak et al, 2016). These results have been interpreted to suggest that 5-HT2C agonists might have utility as candidate cocaine use disorder pharmacotherapies, but it should be noted that lorcaserin treatments that decreased cocaine self-administration also decreased food-maintained responding in monkeys. Poor selectivity of treatment effects on cocaine- vs food-maintained responding in preclinical studies is often associated with poor treatment outcomes in clinical trials (Mello and Negus, 1996).
In contrast to 5-HT2C receptors, 5-HT2A receptors are located primarily on dopamine neurons, and as a result, attenuation of dopamine neuronal activity can theoretically be achieved not by stimulating 5-HT2A receptors, but by inhibiting them. Consistent with this possibility, intra-accumbens pretreatment with the 5-HT2A antagonist M100,907 attenuated cocaine-induced NAc extracellular dopamine increases in the rat (Zayara et al, 2011). Systemic M100,907 failed to attenuate cocaine-induced NAc extracellular dopamine increases, but did attenuate cocaine-induced striatal dopamine increases in monkeys (Murnane et al, 2013). Furthermore, pretreatment with M100,907 (Fletcher et al, 2002; McMahon and Cunningham, 2001; Murnane et al, 2013) or another 5-HT2A antagonist ketanserin (Munzar et al, 2002) attenuated the discriminative stimulus and reinstating effects of cocaine in both rats and monkeys. Although acute 5-HT2A antagonist pretreatments have failed to attenuate cocaine self-administration in both rats (Filip, 2005; Fletcher et al, 2002; Nic Dhonnchadha et al, 2009) and monkeys (Fantegrossi et al, 2002; Murnane et al, 2013), repeated 5-HT2A antagonist treatment effects on cocaine self-administration are unknown.
The study goal was to further evaluate the potential of 5-HT2 receptor ligands as candidate medications for cocaine use disorder. Specifically, this study examined repeated 7-day treatment effects with the 5-HT2C agonist lorcaserin or the 5-HT2A inverse agonist/antagonist pimavanserin, administered alone or in combination, on cocaine self-administration by rhesus monkeys in the context of a cocaine-vs-food choice procedure that has been used previously to examine effects of other candidate medications (Banks et al, 2015). Lorcaserin was investigated because it is more selective for 5-HT2C vs 5-HT2A than RO 60-0175 (Bentley et al, 2004; Thomsen et al, 2008) and because it has been approved by the U.S. Food and Drug Administration (FDA) for obesity treatment (Shukla et al, 2015). Pimavanserin was investigated because it is a 5-HT2A antagonist/inverse agonist (Vanover et al, 2006) that has received FDA approval for Parkinson’s disease-induced psychosis treatment (Walsh, 2016). The combination was evaluated because recent evidence suggests a potential for synergism in 5-HT2C agonist and 5-HT2A antagonist effects (Cunningham et al, 2013). Repeated treatment effects were determined because pharmacotherapies for substance use disorders are typically administered chronically in the clinic, and repeated treatment regimens enhance preclinical-to-clinical translation (Comer et al, 2008; Czoty et al, 2016; Haney and Spealman, 2008; Mello and Negus, 1996). A cocaine vs food choice procedure was used for two reasons. First, choice procedures provide a measure of behavioral allocation that permits dissociation of treatment effects on the relative reinforcing effectiveness of cocaine from nonselective effects on operant responding (Banks and Negus, 2012). Second, preclinical choice procedures promote preclinical-to-clinical translation of results, both because human laboratory studies also commonly use choice procedures to assess candidate medication effects on cocaine self-administration, and because a clinical goal of treatment is both to reduce cocaine use and to increase more adaptive behaviors maintained by nondrug reinforcers (Comer et al, 2008; Haney and Spealman, 2008; Vocci, 2007). On the basis of the preclinical neurochemical and behavioral evidence cited above, we hypothesized that repeated lorcaserin and pimavanserin treatment would attenuate cocaine choice and produce a complementary increase in food choice.
Materials and methods
Subjects
Studies were conducted in a total of eight adult male rhesus monkeys (Macaca mulatta) of either Indian or Chinese origin. Three monkeys were used in studies of schedule-controlled responding for food delivery, and five monkeys were surgically implanted with a double-lumen venous catheter (STI Components, Raleigh, NC or Reiss Manufacturing, Blackstone, VA) for studies of cocaine vs food choice. Of these five catheterized monkeys, three had prior cocaine self-administration histories, and two had prior methamphetamine discrimination histories. Monkeys could earn 1 g banana-flavored pellets (Grain-based Precision Primate Tablets; Test Diets, Richmond, IL) during daily experimental sessions (see below). The monkeys diet consisted of food biscuits (Lab Diet High Fiber Monkey Biscuits; PMI Feeds, St Louis, MO) and fresh fruit delivered in the afternoons after behavioral sessions to minimize the effects of biscuit availability and consumption on food-maintained operant responding. Water was continuously available in each monkey’s home chamber, which also served as the experimental chamber. A 12 h light/dark cycle was in effect (lights on from 0600 to 1800 h). Environmental enrichment, which consisted of movies displayed on a monitor in the housing room and foraging boards loaded with nuts, seeds, or diced vegetables was also provided after behavioral sessions. Facilities were licensed by the United States Department of Agriculture and accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. The Institutional Animal Care and Use Committee approved all experimental and enrichment protocols. Animal research and husbandry were conducted according to the eighth edition of the Guide for the Care and Use of Laboratory Animals.
Assay of Schedule-Controlled Responding
Behavioral procedure
To provide an initial assessment of the potency and time course of intramuscular (IM) lorcaserin and pimavanserin in rhesus monkeys, experiments were conducted in a food-maintained schedule-controlled responding procedure. As described previously (Banks et al, 2010), each home chamber was equipped with a customized operant response panel, which had a response key that could be transilluminated red, and a pellet dispenser (Med Associates, ENV-203–1000, St. Albans, VT) that delivered food pellets to a receptacle below the operant panel. The panel was controlled by a MED-PC interface and an IBM compatible computer programmed in MEDSTATE Notation (Med Associates). Experimental sessions were 150 min in duration and consisted of five 30 min cycles. Each cycle consisted of two components: a 25 min time-out period followed by a 5 min response period. During the time-out period, no stimulus lights were illuminated, and responding had no scheduled consequences. During the response period, the right key was transilluminated red and subjects could respond for up to 10 food pellets under a fixed-ratio 30 (FR30) schedule of reinforcement. If all 10 food pellets were earned before 5 min had elapsed, the lights were turned off and responding had no scheduled consequences for the remainder of that response period. All monkeys were trained until they responded at relatively stable rates≥1.0 response per second during all five cycles for 10 consecutive days (data not shown).
Behavioral sessions were conducted 5 days a week. Test sessions were usually conducted on Tuesdays and Fridays, and training sessions were conducted on Mondays, Wednesdays, and Thursdays. In addition, test sessions were conducted only after a training session during which the monkeys responded at rates ≥1.0 response per second for all five cycles. During training sessions, monkeys received either no injection or saline injections at the beginning of each cycle. During time course test sessions, either saline, lorcaserin (0.1–1.0 mg/kg, IM) or pimavanserin (1.0–10.0 mg/kg, IM) was administered, and 5 min response periods were initiated 10, 30, 100, 130, 300 min, and 24 h after administration.
Data analysis
Raw response rates from each test cycle were converted to percent of control using the average rate from the previous training day in that monkey as the control value. data were analyzed (JMP Pro 12.2.0, SAS, Cary, NC) using a two-way repeated-measures ANOVA with drug dose and time as the main factors. In the presence of a significant main effect of drug dose or drug dose × time interaction, post hoc analyses were conducted using the Dunnett post hoc test for comparisons with saline within a given time point. The criterion for significance was set a priori at the 95% confidence level (P<0.05).
Assay of Cocaine vs Food Choice
Behavioral procedure
As described previously (Banks et al, 2011), each housing chamber was equipped with a customized operant response panel, which had two response keys that could be transilluminated red or green, and a pellet dispenser (Med Associates, ENV-203–1000) that delivered food pellets to a receptacle below the operant panel. The externalized portion of the intravenous (IV) catheter was routed through a custom jacket and tether system connected to a dual-channel fluid swivel (Lomir Biomedical, Malone, NY) on the chamber top and then to two safety syringe pumps (Med Associates, PHM-108), one for each lumen of the double-lumen catheter. One pump was used to deliver contingent cocaine injections through one lumen of the double-lumen catheter. The second pump was used to deliver noncontingent saline or lorcaserin (0.032-0.1 mg/kg/h) injections through the second lumen at a programmed rate of 0.1 ml injections every 20 min from 1200 h each day until 1100 h the next morning. Catheter patency was periodically evaluated with IV ketamine (Vedco, St Joseph, MO) administration and after any treatment that produced a rightward shift in the cocaine choice dose-effect function. The catheter was considered patent if IV ketamine administration produced muscle tone loss within 10 s.
Daily experimental sessions were conducted from 0900 to 1100 h in each monkey’s home chamber as described previously (Banks et al, 2011). The terminal choice schedule consisted of five 20 min components separated by 5 min inter-component intervals, during which responding had no scheduled consequences. During each component, the left, food-associated key was transilluminated red, and completion of the FR requirement (FR100) resulted in food pellet delivery. In addition, the right, cocaine-associated key was transilluminated green, and completion of the FR requirement (FR10) resulted in delivery of the IV unit cocaine dose available during that component. The unit cocaine doses available during each of the five successive components were 0, 0.0032, 0.01, 0.032, and 0.1 mg/kg per injection, respectively. Stimulus lights on the cocaine-associated key were flashed on and off in 3 s cycles, and longer flashes were associated with higher cocaine doses. Ratio requirement completion initiated a 3 s timeout, during which all stimulus lights were turned off, and responding had no scheduled consequences. Experimental parameters used in this study were based on extensive parametric manipulations (Banks et al, 2013b; Negus, 2003) and permitted detection of both leftward and rightward shifts in the cocaine choice dose-effect function. Choice behavior was considered to be stable when the lowest unit cocaine dose maintaining at least 80% cocaine vs food choice varied by ≤0.5 log units for 3 consecutive days. The data from these 3 days were subsequently used as the ‘baseline’ for statistical and graphical comparisons with each pharmacological treatment.
Once cocaine vs food choice was stable, experimental test periods were conducted to determine lorcaserin or pimavanserin treatment effects on cocaine vs food choice. Each dose of lorcaserin (0.1–1.0 mg/kg per day IM) or pimavanserin (0.32–10 mg/kg per day IM) was tested by repeated administration for seven consecutive days. IM lorcaserin doses were administered between 0840 and 0850 h, and IM pimavanserin doses were administered between 0755 and 0805 h, before the start of the 0900 h behavioral choice session. At the conclusion of each 7-day treatment period, treatments were terminated for at least 4 days and until cocaine vs food choice had returned to pretest levels. Each drug treatment was evaluated in a cohort of four monkeys, and three monkeys received both drugs alone and the combination. The dose order within each drug was counterbalanced across subjects, and in those monkeys that received both lorcaserin and pimavanserin, pimavanserin was tested first. The largest pimavanserin dose (10 mg/kg per day) produced large decreases in food-maintained responding, biscuit consumption, or body weights so this dose was tested in only two monkeys. A follow-up study evaluated effects of combined treatment with the largest inactive doses of repeated lorcaserin (0.1 mg/kg) and pimavanserin (0.32 mg/kg) in four monkeys.
Data analysis
The primary-dependent measures for each component were percent cocaine choice, defined as (number of ratio requirements, or ‘choices’, completed on the cocaine-associated key/total number of ratio requirements completed on both the cocaine- and food-associated keys) × 100 and rates of operant responding in responses per second. Mean data from the 3 days preceding each pharmacological treatment were averaged for each individual monkey and then averaged across monkeys to yield the group mean ‘baseline’ data. Mean data from the last 3 days of each 7-day lorcaserin, pimavanserin, or lorcaserin+pimavanserin treatment were averaged for each individual monkey and then averaged across monkeys to yield the group mean data. Percent cocaine choice was then plotted as a function of the unit cocaine dose and analyzed using a mixed-model analysis (JMP Pro 12, SAS) with treatment drug-dose and unit cocaine dose as the fixed main effects and subject as the random effect. A Dunnett’s test was performed to compare treatment effects with baseline within a unit cocaine dose. Additional dependent measures collected during each behavioral session included the numbers of food, cocaine, and total choices summed across all components, and these data were analyzed using one- or two-way repeated-measures ANOVA and a Dunnett’s test as appropriate. The criterion for significance was set a priori at the 95% confidence level (P<0.05).
Drugs
(−)-Cocaine HCl, lorcaserin HCl, and pimavanserin L-tartrate were provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). All drugs were dissolved in sterile water, and all solutions were passed through a 0.22 micron sterile filter (Millipore, Billerica, MA) before either intravenous or intramuscular administration. Drug doses were calculated and expressed using the salt forms listed above.
Results
Lorcaserin and Pimavanserin Effects on Schedule-Controlled Responding
The average±SEM control rate of responding throughout the study was 2.44±0.24 responses/s. Both lorcaserin (dose: F3,40=31.3, P<0.0001; interaction: F11,40=4.0, P=0.006) and pimavanserin (dose: F1.03,2.07=66.4, P=0.0133) significantly decreased rates of operant responding (Figure 1). For lorcaserin, both 0.32 and 1.0 mg/kg significantly decreased response rates, and the time course of these lorcaserin effects were dose dependent and dissipated by 5 h (Figure 1a). Pimavanserin had a slow onset of action, and a significant decrease in response rates was not observed until 24 h after 10 mg/kg pimavanserin (Figure 1b). Response rates were not evaluated again until 72 h after pimavanserin treatment, and by this time, rates recovered to baseline levels (data not shown). On basis of these behavioral results and human pimavanserin neuroimaging and pharmacokinetic data (Nordstrom et al, 2008), lorcaserin was administered as a 15 min pretreatment and pimavanserin was administered as a 60 min pretreatment for the cocaine vs food choice experiments.
Potency and time course of acute (a) lorcaserin (0.1–1.0 mg/kg, intramuscular) and (b) pimavanserin (1.0–10.0 mg/kg, intramuscular) effects in a schedule-controlled responding procedure in rhesus monkeys (n=3). Abscissae: time in minutes (min) or hours (h) after drug administration. Ordinates: percent control rate of responding. All points represent mean±SEM. Filled symbols denote significantly different from saline (p<0.05).
Because the IM lorcaserin time course effects on food-maintained responding were dissipating by the 135 min time point, a time point that corresponded with fourth and fifth components of the cocaine vs food choice session where cocaine was primarily chosen over food, a follow-up study was conducted evaluating 7-day treatment effects of lorcaserin (0.032–0.1 mg/kg/h) using a continuous intravenous delivery procedure previously described by our laboratory (Banks et al, 2013a; Negus, 2004). Due to limited lorcaserin supply, 0.1 mg/kg/h lorcaserin was tested in four monkeys and 0.032 mg/kg/h lorcaserin was tested in two monkeys. At the conclusion of each 7-day treatment period, saline treatment conditions were instituted for at least 4 days and until cocaine vs food choice had returned to pretest levels. Lorcaserin doses were evaluated in descending order. Pimavanserin was not evaluated under continuous intravenous delivery conditions due to the long and protracted time course of effects in the assay of schedule-controlled responding.
Lorcaserin Effects on Cocaine Choice
Under baseline conditions, monkeys primarily chose food when cocaine was not available (0 mg/kg per injection) or the unit cocaine dose was small (0.0032–0.01 mg/kg per injection) and almost exclusively reallocated their behavior to cocaine choice during availability of larger unit cocaine doses (0.032–0.1 mg/kg per injection) (Figures 2,3,4; dashed lines). Figure 2 shows 7-day repeated lorcaserin (0.1–1.0 mg/kg per day) and (0.032–0.1 mg/kg/h) treatment effects on cocaine choice (A, D), rates of operant responding per component (B, E), and numbers of total, food, and cocaine choices per session (C, F), respectively. Because 0.032 mg/kg/h lorcaserin was only tested in two monkeys, statistical analyses were only conducted on the 0.1 mg/kg/h lorcaserin data. Repeated IM or IV lorcaserin treatment did not significantly alter percent cocaine choice. Both 1.0 mg/kg per day (lorcaserin: F3,9=13.2, p=0.0012) and 0.1 mg/kg/h (lorcaserin: F1,3=17.7, p=0.0245; interaction: F3,9=5.0, p=0.0267) lorcaserin significantly decreased rates of operant responding. 1.0 mg/kg per day lorcaserin also significantly decreased both total and food choices per session (2C; dependent measure: F2,24=74.7, p<0.0001; lorcaserin dose: F3,12=12.7, p=0.0005; interaction: F6,24=4.9, p=0.002).
Effects of 7-day intramuscular (0.1–1.0 mg/kg per day, IM; a–c) or intravenous (0.032–0.1 mg/kg/h, IV; d–f) lorcaserin treatment on choice between cocaine and food in rhesus monkeys. Intramuscular (IM) lorcaserin was administered daily 15 min before the behavioral session. Top and middle abscissae: unit dose cocaine in mg/kg per injection. Top ordinate: percent cocaine choice. Middle ordinate: rates of operant responding per component in responses per second. Bottom abscissa: dependent measure. Bottom ordinate: number of choices per session. All points and bars represent mean±SEM obtained during days 5–7 of each 7-day treatment period. Filled symbols in top panels, and * in the bottom panel, denote significantly different from baseline (p<0.05). IM lorcaserin and 0.1 mg/kg/h IV lorcaserin were evaluated in 4 monkeys. 0.032 mg/kg/h IV lorcaserin was evaluated in 2 monkeys.
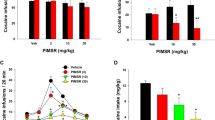
Effects of 7-day intramuscular pimavanserin (0.32–3.2 mg/kg per day, IM) treatment on choice between cocaine and food in rhesus monkeys (n=4). IM pimavanserin was administered daily 60 min before the behavioral session. Top and middle abscissae: unit dose cocaine in mg/kg per sinjection. Top ordinate (a): percent cocaine choice. Middle ordinate (b): rates of responding per component in responses per second. Bottom abscissa: dependent measure. Bottom ordinate (c): number of choices per session. All points and bars represent mean±SEM obtained during days 5–7 of each 7-day treatment period. Numbers in parentheses indicate the number of monkeys contributing to that data point if fewer than the total number (n=4) of monkeys tested and denote a component of the behavioral procedure in which one or more monkeys failed to complete at least one response requirement. Filled symbols in top panel, or * in the bottom panel, denote significantly different from baseline (p<0.05).
Effects of 7-day pimavanserin (10 mg/kg per day, IM) treatment on choice between cocaine and food in individual rhesus monkeys. IM pimavanserin was administered daily 60 min before the behavioral session. Top and middle abscissae: unit dose cocaine in mg/kg/injection. Top ordinates (a, d): percent cocaine choice. Middle ordinates (b, e): rates of operant responding per component in responses per second. Bottom abscissae: dependent measure. Bottom ordinates (c, f): number of choices per session. All points represent mean data obtained during days 5–7 of each 7-day treatment period. Error bars are not shown due to individual subject data.
Pimavanserin Effects on Cocaine Choice
Figure 3 shows 7-day repeated pimavanserin treatment effects on cocaine choice (A), rates of operant responding per component (B), and the overall numbers of total, food, and cocaine choices for the entire session (C). Pimavanserin produced a dose-dependent increase in cocaine choice and a decrease in the numbers of total and food choices per session. Repeated 3.2 mg/kg per day pimavanserin significantly increased preference for smaller unit cocaine doses (0.0032–0.01 mg/kg/injection) (cocaine: F3,41=36.2, p<0.0001; pimavanserin: F3,41.6=4.0, p=0.013; interaction: F9,41.2=3.0, p=0.0071). Furthermore, repeated 1.0 and 3.2 mg/kg per day pimavanserin significantly decreased the numbers of both total and food choices per session (dependent measure: F2,6=149.7, p<0.0001; interaction: F6,18=3.0, p=0.0324). Repeated 10 mg/kg per day pimavanserin eliminated food-maintained responding during concurrent cocaine availability such that both monkeys exclusively chose cocaine over food when they responded (Figure 4).
Pimavanserin and Lorcaserin Coadministration Effects on Cocaine Choice
Figure 5 shows 7-day repeated 0.32 mg/kg per day pimavanserin+0.1 mg/kg per day lorcaserin coadministration treatment effects on cocaine choice (A), rates of operant responding per component (B), and numbers of total food, and cocaine choices per session (C). This combination of pimavanserin and lorcaserin doses did not significantly alter any experimental dependent measure.
Effects of 7-day combined intramuscular pimavanserin (0.32 mg/kg, IM)+lorcaserin (0.1 mg/kg, IM) treatment on choice between cocaine and food in rhesus monkeys (n=4). IM pimavanserin and lorcaserin were administered daily 60 min and 15 min, respectively before the behavioral session.Top and middle abscissae: unit dose cocaine in mg/kg per injection. Top ordinate (a): percent cocaine choice. Middle ordinate (b): rates of operant responding per component in responses per second. Bottom abscissa: dependent measure. Bottom ordinate (c): number of choices per session. All points and bars represent mean±SEM obtained during days 5–7 of each 7-day treatment period.
Discussion
The present study examined repeated 7-day treatment effects with either the 5-HT2C agonist lorcaserin or the 5-HT2A inverse agonist/antagonist pimavanserin, administered alone or in combination, on cocaine vs food choice in rhesus monkeys. There were three main findings. First, both acute and repeated lorcaserin and pimavanserin treatment dose-dependently decreased rates of operant responding in monkeys. Second, both lorcaserin and pimavanserin failed to decrease cocaine vs food choice at doses at or below those that decreased overall rates of operant responding and reinforcement. Finally, a single combination of ineffective lorcaserin and pimavanserin doses failed to alter cocaine choice, rates of responding, or rates of reinforcement. Overall, the present results do not support the clinical utility of either 5-HT2C agonists or 5-HT2A inverse agonists/antagonists alone or in combination as candidate anti-cocaine use disorder pharmacotherapies.
Lorcaserin Effects on Cocaine Choice
5-HT2C agonists in general, and lorcaserin in particular, have recently emerged as intriguing candidate medications for cocaine use disorder treatment. Enthusiasm for 5-HT2C agonists stems in part from evidence in rats (Cunningham et al, 2011; Fletcher et al, 2008; Grottick et al, 2000; Harvey-Lewis et al, 2016) and nonhuman primates (Collins et al, 2016; Gerak et al, 2016; Manvich et al, 2012) that 5-HT2C agonists produce dose-dependent decreases in cocaine self-administration after acute treatment and sustained decreases in cocaine self-administration during repeated treatment for periods up to 14 days. A caveat to these results has been that 5-HT2C agonist doses that decrease cocaine self-administration also usually decrease responding maintained by other reinforcers. For example, RO 60-0175 was equipotent to decrease responding maintained by 0.25 mg/injection cocaine and 45 mg food pellets in rats (Grottick et al, 2000), and lorcaserin was equipotent to decrease responding maintained by 0.032 mg/kg per injection cocaine and 300 mg food pellets in rhesus monkeys (Collins et al, 2016). In squirrel monkeys, RO 60-0175 was slightly (<3-fold) more potent to decrease self-administration maintained by a range of cocaine doses than by termination of a stimulus associated with shock delivery; however, another 5-HT2C agonist, mCPP, did not display this selectivity, and in rats, the 5-HT2C agonist WAY 163909 was actually less potent to decrease cocaine self-administration than sucrose-maintained responding (Cunningham et al, 2011; Manvich et al, 2012). Taken together, these results suggest that 5-HT2C agonists do not selectively decrease the reinforcing effectiveness of cocaine, but rather produce nonselective decreases in operant responding maintained by a broad range of reinforcers.
In agreement with these previous studies, the present study found that lorcaserin produced a dose-dependent, but nonselective, decrease in responding maintained by cocaine and food. Additionally, the failure of lorcaserin to decrease measures of cocaine-vs-food choice provides additional evidence to suggest that lorcaserin did not decrease the relative reinforcing efficacy of cocaine in comparison with food. This profile of nonselective decreases in operant responding and failure to decrease cocaine-vs-food choice in preclinical studies has generally predicted poor outcomes in clinical trials (Banks et al, 2015; Czoty et al, 2016; Mello and Negus, 1996). As a result, we interpret the present and previous results as evidence against the hypothesis that 5-HT2C agonists will have utility for cocaine use disorder treatment.
Insofar as behavioral selectivity to decrease cocaine self-administration and cocaine choice is a desirable attribute of candidate medications, it important to note that expression of behavioral selectivity can be influenced by factors independent of the mediction, such as the unit cocaine dose and the type and magnitude of the alternative reinforcer. Regarding cocaine dose, for example, lorcaserin was equipotent (ie, nonselective) to decrease responding maintained by 0.032 mg/kg per injection cocaine and 300 mg food pellets in rhesus monkeys, but even the highest lorcaserin-doses tested in that study failed to decrease self-administration of higher cocaine doses (0.1–0.32 mg/kg per injection) (Collins et al, 2016; Gerak et al, 2016). More generally, the behavioral selectivity of candidate medications or other treatments to decrease cocaine self-administration or cocaine choice is often inversely related to the unit cocaine dose, with greater selectivity at lower cocaine doses (Banks et al, 2013b; Mello and Negus, 1996; Stafford et al, 2000). Medications that display behavioral selectivity to decrease large- as well as small-dose cocaine self-administration might be optimal, in part, because drug abuse typically involves the use of large doses and also because responding maintained by small doses can often be reduced by nonpharmacological strategies (eg, contingency management) (Donny et al, 2003; Lile et al, 2016).
Preclinical measures of behavioral selectivity can also be influenced by parameters of the alternative reinforcer. Of particular relevance for this study, lorcaserin is clinically approved as an anorectic medication for treatment of obesity, and it has established effectiveness to reduce food consumption (Shukla et al, 2015). This raises the possibility that lorcaserin treatment decreased the relative reinforcing effectiveness of food and cocaine equally, resulting in a net no change in cocaine-vs-food choice behavior. An implication of this alternative explanation would be that behavioral selectivity of lorcaserin effects on cocaine self-administration might improve if an alternative reinforcer other than food were used. Some support for this possibility is provided by the comparison of 5-HT2C agonist effects on responding maintained in squirrel monkeys by cocaine and by termination of a stimulus associated with shock delivery (Manvich et al, 2012). However, even in this study, effects of one 5-HT2C agonist (mCPP) were nonselective, and effects of the other (RO 60-0175) were only weakly selective. Additionally, the failure of lorcaserin to decrease cocaine-vs-food choice in the present study contrasts with the effectiveness of other clinically approved anorectic drugs (eg, d-amphetamine and phendimetrazine) to decrease cocaine-vs-food choice under identical conditions (Banks et al, 2011; Banks et al, 2013a). These results suggest that evidence of poor selectivity for 5-HT2C agonists to decrease cocaine self-administration and cocaine choice cannot be attributed solely to use of food as the alternative reinforcer. More generally, the present results extend the range of conditions under which 5-HT2C agonists fail to produce selective decreases in cocaine self-administration.
Pimavanserin and Lorcaserin+Pimavanserin Effects on Cocaine Choice
Repeated treatment with the 5-HT2A inverse agonist/antagonist pimavanserin also failed to attenuate cocaine choice and produce a complementary increase in food choice up to pimavanserin doses that disrupted rates of responding. The present results confirm and extend previous findings that acute 5-HT2A antagonist pretreatment does not attenuate cocaine self-administration in either rats (Filip, 2005; Fletcher et al, 2002; Nic Dhonnchadha et al, 2009) or monkeys (Fantegrossi et al, 2002; Murnane et al, 2013). Thus, repeated pimavanserin treatment in the present study did not reveal a therapeutic-like rightward shift in the cocaine choice dose-effect function. Furthermore, the present results are also consistent with repeated quetiapine (Brutcher and Nader, 2015) and risperidone (Hutsell et al, 2016) treatment effects on cocaine choice. These two atypical antipsychotics possess 5-HT2A antagonist properties (Richelson and Souder, 2000), and like pimavanserin, they also failed to decrease cocaine vs food choice in rhesus monkeys. Overall, the present results and the extant preclinical literature suggest that 5-HT2A receptors are not necessary for cocaine reinforcement and that 5-HT2A antagonists do not represent a promising class of anti-cocaine use disorder medications.
One potential strategy to reduce undesirable effects and enhance the therapeutic effects of canidate cocaine use disorder medications might be to combine 5-HT2c agonists and 5-HT2A antagonist treatments (Howell and Cunningham, 2015). For example, the combination of ineffective 5-HT2c agonist (WAY163909) and 5-HT2A antagonist (M100,907) doses significantly decreased both cocaine- and cue-primed reinstatement of extinguished cocaine self-administration (Cunningham et al, 2013). In contrast, the present study found no evidence of a therapeutic-like treatment effect on cocaine choice by combining lorcaserin and pimavanserin doses that alone did not significantly alter rates of operant responding during repeated administration. Larger-dose combinations were not tested due to nonselective decreases in rates of operant responding as reported above. However, the degree to which repeated treatment with other lorcaserin and pimavanserin dose combinations might alter cocaine choice remains to be empirically determined.
Implications for Anti-Cocaine Addiction Medication Development
Currently, there are two ongoing human-laboratory studies (NCT02680288 and NCT02537873) evaluating acute and repeated lorcaserin treatment effects on cocaine self-administration in humans. Results of these and other related studies will help to clarify both the utility of lorcaserin to treat cocaine use disorder and the profile of preclinical effects in laboratory animals that is most predictive of candidate medication effects in humans. Notably, both studies are evaluating lorcaserin effects on choice between intravenous cocaine doses and monetary alternative reinforcers, and the predominant use of choice procedures in these types of human-laboratory studies is one rationale for the use of choice procedures in preclinical studies such as the one reported here.
Further consideration of lorcaserin and pimavanserin as treatment options for cocaine use disorder will have to await the outcome of human-laboratory studies and clinical trials, but the present results can also be compared with results of other candidate medications that have already progressed through both preclinical and clinical studies. In particular, lorcaserin and pimavanserin were tested here as candidate medications hypothesized to reduce cocaine choice by reducing cocaine effects on mesolimbic dopamine release. In this regard, the effects of these 5-HT2 receptor ligands can be compared with effects of other medications hypothesized to attenuate cocaine-induced stimulation of mesolimbic dopamine transmission, including dopamine receptor antagonists (which block postsynaptic dopamine receptors) and kappa opioid receptor agonists (which activate inhibitory kappa receptors on mesolimbic dopamine neurons to inhibit dopamine release). Like lorcaserin and pimavanserin, both dopamine receptor antagonists (John et al, 2015; Negus et al, 1996) and selective kappa receptor agonists (Negus, 2004; Negus et al, 1997) produce nonselective decreases in cocaine- vs food-maintained responding and fail to decrease cocaine-vs-food choice in rhesus monkeys. Moreover, both dopamine receptor antagonists and kappa receptor agonists have failed to reduce cocaine use in human-laboratory studies and/or clinical trials (Grabowski et al, 2000; Walsh et al, 2001; Winhusen et al, 2014). Overall, then, the results of the present study with lorcaserin and pimavanserin are consistent with other evidence from other drug classes that pharmacological inhibition of mesolimbic dopamine transmission is not an effective approach to treatment of cocaine use disorder. Additionally, the poor effectiveness of these compounds contrasts with the effectiveness of some other approaches, such as amphetamine maintenance, to produce sustained and selective decreases in cocaine self-administration and cocaine-vs-food choice in rats and rhesus monkeys and to decrease metrics of cocaine use in both human-laboratory studies and clinical trials (Banks et al, 2015; Pérez-Mañá et al, 2011).
Funding and disclosure
Research reported in this article was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number R01 DA026946. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Both Banks and Negus declare that NIH has funded their research. The authors declare no conflict of interest.
References
Acri J, Skolnick P (2013). Pharmacotherapy of substance use disorders. In: Charney D, Buxbaum J, Sklar P, Nestler EJ (eds). Neurobiology of Mental Illness, Oxford University Press: London, UK, 2013, pp 761–771.
Alex KD, Pehek EA (2007). Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther 113: 296–320.
Banks ML, Blough BE, Negus SS (2011). Effects of monoamine releasers with varying selectivity for releasing dopamine/norepinephrine versus serotonin on choice between cocaine and food in rhesus monkeys. Behav Pharmacol 22: 824–836.
Banks ML, Blough BE, Negus SS (2013a). Effects of 14-day treatment with the schedule III anorectic phendimetrazine on choice between cocaine and food in rhesus monkeys. Drug Alcohol Depend 131: 204–213.
Banks ML, Blough BE, Negus SS (2013b). Interaction between behavioral and pharmacological treatment strategies to decrease cocaine choice in rhesus monkeys. Neuropsychopharmacology 38: 395–404.
Banks ML, Hutsell BA, Schwienteck KL, Negus SS (2015). Use of preclinical drug vs. food choice procedures to evaluate candidate medications for cocaine addiction. Curr Treat Options Psychiatry 2: 136–150.
Banks ML, Negus SS (2012). Preclinical determinants of drug choice under concurrent schedules of drug self-administration. Adv Pharmacol Sci 2012: 281768.
Banks ML, Rice KC, Negus SS (2010). Antinociceptive interactions between mu-opioid receptor agonists and the serotonin uptake inhibitor clomipramine in rhesus monkeys: role of mu agonist efficacy. J Pharmacol Exp Ther 335: 497–505.
Bentley JM, Adams DR, Bebbington D, Benwell KR, Bickerdike MJ, Davidson JEP et al (2004). Indoline derivatives as 5-HT2C receptor agonists. Bioorg Med Chem Lett 14: 2367–2370.
Brutcher RE, Nader MA (2015). Effects of quetiapine treatment on cocaine self-administration and behavioral indices of sleep in adult rhesus monkeys. Psychopharmacology 232: 411–420.
Callahan PM, Cunningham KA (1995). Modulation of the discriminative stimulus properties of cocaine by 5-HT1B and 5-HT2C receptors. J Pharmacol Exp Ther 274: 1414–1424.
Cathala A, Devroye C, Maitre M, Piazza PV, Abrous DN, Revest J-M et al (2015). Serotonin2C receptors modulate dopamine transmission in the nucleus accumbens independently of dopamine release: behavioral, neurochemical and molecular studies with cocaine. Addict Biol 20: 445–457.
Collins GT, Gerak LR, Javors MA, France CP (2016). Lorcaserin reduces the discriminative stimulus and reinforcing effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther 356: 85–95.
Comer SD, Ashworth JB, Foltin RW, Johanson CE, Zacny JP, Walsh SL (2008). The role of human drug self-administration procedures in the development of medications. Drug Alcohol Depend 96: 1–15.
Cunningham KA, Anastasio NC, Fox RG, Stutz SJ, Bubar MJ, Swinford SE et al (2013). Synergism between a serotonin 5-HT2A receptor (5-HT2AR) antagonist and 5-HT2CR agonist suggests new pharmacotherapeutics for cocaine addiction. ACS Chem Neurosci 4: 110–121.
Cunningham KA, Fox RG, Anastasio NC, Bubar MJ, Stutz SJ, Moeller FG et al (2011). Selective serotonin 5-HT2C receptor activation suppresses the reinforcing efficacy of cocaine and sucrose but differentially affects the incentive-salience value of cocaine- vs. sucrose-associated cues. Neuropharmacology 61: 513–523.
Czoty PW, Stoops WW, Rush CR (2016). Evaluation of the “pipeline” for development of medications for cocaine use disorder: A review of translational preclinical, human laboratory, and clinical trial research. Pharmacol Rev 68: 533–562.
Donny EC, Bigelow GE, Walsh SL (2003). Choosing to take cocaine in the human laboratory: effects of cocaine dose, inter-choice interval, and magnitude of alternative reinforcement. Drug Alcohol Depend 69: 289–301.
Fantegrossi WE, Ullrich T, Rice KC, Woods JH, Winger G (2002). 3,4-Methylenedioxymethamphetamine (MDMA, “ecstasy”) and its stereoisomers as reinforcers in rhesus monkeys: serotonergic involvement. Psychopharmacology 161: 356–364.
Filip M (2005). Role of serotonin (5-HT)2 receptors in cocaine self-administration and seeking behavior in rats. Pharmacol Rep 57: 35–46.
Fletcher PJ, Grottick AJ, Higgins GA (2002). Differential effects of the 5-HT2A receptor antagonist M100,907 and the 5-HT2C receptor antagonist SB242,084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology 27: 576–586.
Fletcher PJ, Rizos Z, Sinyard J, Tampakeras M, Higgins GA (2008). The 5-HT2C receptor agonist Ro60-0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine, and contextual cues. Neuropsychopharmacology 33: 1402–1412.
Gerak LR, Collins GT, France CP (2016). Effects of lorcaserin on cocaine and methamphetamine self-administration and reinstatement of extinguished responding in rhesus monkeys. J Pharmacol Exp Ther 359: 383–391.
Grabowski J, Rhoades H, Silverman P, Schmitz JM, Stotts A, Creson D et al (2000). Risperidone for the treatment of cocaine dependence: randomized, double-blind trial. J Clin Psychopharmacol 20: 305–310.
Grottick AJ, Fletcher PJ, Higgins GA (2000). Studies to investigate the role of 5-HT2C receptors on cocaine- and food-maintained behavior. J Pharmacol Exp Ther 295: 1183–1191.
Haney M, Spealman R (2008). Controversies in translational research: drug self-administration. Psychopharmacology 199: 403–419.
Hannon J, Hoyer D (2008). Molecular biology of 5-HT receptors. Behav Brain Res 195: 198–213.
Harvey-Lewis C, Li Z, Higgins GA, Fletcher PJ (2016). The 5-HT2C receptor agonist lorcaserin reduces cocaine self-administration, reinstatement of cocaine-seeking and cocaine induced locomotor activity. Neuropharmacology 101: 237–245.
Howell LL, Cunningham KA (2015). Serotonin 5-HT2 receptor interactions with dopamine function: Implications for therapeutics in cocaine use disorder. Pharmacol Rev 67: 176–197.
Hutsell BA, Negus SS, Banks ML (2016). Effects of 21-day d-amphetamine and risperidone treatment on cocaine vs food choice and extended-access cocaine intake in male rhesus monkeys. Drug Alcohol Depend 168: 36–44.
John WS, Banala AK, Newman AH, Nader MA (2015). Effects of buspirone and the dopamine D3 receptor compound PG619 on cocaine and methamphetamine self-administration in rhesus monkeys using a food-drug choice paradigm. Psychopharmacology 232: 1279–1289.
Lile JA, Stoops WW, Rush CR, Negus SS, Glaser PEA, Hatton KW et al (2016). Development of a translational model to screen medications for cocaine use disorder II: choice between intravenous cocaine and money in humans. Drug Alcohol Depend 165: 111–119.
Manvich DF, Kimmel HL, Howell LL (2012). Effects of serotonin 2C receptor agonists on the behavioral and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther 341: 424–434.
McMahon LR, Cunningham KA (2001). Antagonism of 5-Hydroxytryptamine2 A receptors attenuates the behavioral effects of cocaine in rats. J Pharmacol Exp Ther 297: 357–363.
Mello NK, Negus SS (1996). Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology 14: 375–424.
Munzar P, Justinova Z, Kutkat SW, Goldberg SR (2002). Differential involvement of 5-HT2A receptors in the discriminative-stimulus effects of cocaine and methamphetamine. Eur J Pharmacol 436: 75–82.
Murnane KS, Winschel J, Schmidt KT, Stewart LM, Rose SJ, Cheng K et al (2013). Serotonin 2A receptors differentially contribute to abuse-related effects of cocaine and cocaine-induced nigrostriatal and mesolimbic dopamine overflow in nonhuman primates. J Neurosci 33: 13367–13374.
Navailles S, Moison D, Cunningham KA, Spampinato U (2008). Differential regulation of the mesoaccumbens dopamine circuit by serotonin2C receptors in the ventral tegmental area and the nucleus accumbens: An in vivo microdialysis study with cocaine. Neuropsychopharmacology 33: 237–246.
Negus SS (2003). Rapid assessment of choice between cocaine and food in rhesus monkeys: effects of environmental manipulations and treatment with d-amphetamine and flupenthixol. Neuropsychopharmacology 28: 919–931.
Negus SS (2004). Effects of the kappa opioid agonist U50,488 and the kappa opioid antagonist nor-binaltorphimine on choice between cocaine and food in rhesus monkeys. Psychopharmacology 176: 204–213.
Negus SS, Mello NK, Lamas X, Mendelson JH (1996). Acute and chronic effects of flupenthixol on the discriminative stimulus and reinforcing effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther 278: 879–890.
Negus SS, Mello NK, Portoghese PS, Lin C-E (1997). Effects of kappa opioids on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther 282: 44–55.
Nic Dhonnchadha BÁ, Fox RG, Stutz SJ, Rice KC, Cunningham KA (2009). Blockade of the serotonin 5-ht2a receptor suppresses cue-evoked reinstatement of cocaine-seeking behavior in a rat self-administration model. Behav Neurosci 123: 382–396.
Nordstrom A-L, Mansson M, Jovanovic H, Karlsson P, Halldin C, Farde L et al (2008). PET analysis of the 5-HT2A receptor inverse agonist ACP-103 in human brain. Int J Neuropsychopharmacol 11: 163.
Pérez-Mañá C, Castells X, Vidal X, Casas M, Capellà D (2011). Efficacy of indirect dopamine agonists for psychostimulant dependence: a systematic review and meta-analysis of randomized controlled trials. J Subst Abuse Treat 40: 109–122.
Richelson E, Souder T (2000). Binding of antipsychotic drugs to human brain receptors: Focus on newer generation compounds. Life Sci 68: 29–39.
Rüedi-Bettschen D, Spealman RD, Platt DM (2015). Attenuation of cocaine-induced reinstatement of drug seeking in squirrel monkeys by direct and indirect activation of 5-HT2C receptors. Psychopharmacology 232: 2959–2968.
Shukla AP, Kumar RB, Aronne LJ (2015). Lorcaserin HCl for the treatment of obesity. Expert Opin Pharmacother 16: 2531–2538.
Stafford D, Rice KC, Lewis DB, Glowa JR (2000). Response requirements and unit dose modify the effects of GBR 12909 on cocaine-maintained behavior. Exp Clin Psychopharmacol 8: 539–548.
Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D et al (2008). Lorcaserin, a novel selective human 5-Hydroxytryptamine2C agonist: In vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther 325: 577–587.
Vanover KE, Weiner DM, Makhay M, Veinbergs I, Gardell LR, Lameh J et al (2006). Pharmacological and behavioral profile of N-(4-Fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N′-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-Hydroxytryptamine2A receptor inverse agonist. J Pharmacol Exp Ther 317: 910–918.
Vocci FJ (2007). Can replacement therapy work in the treatment of cocaine dependence? and what are we replacing anyway? Addiction 102: 1888–1889.
Walsh S (2016). FDA approves first drug to treat hallucinations and delusions associated with Parkinson's disease. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm498442.htm (accessed on May 6 2016).
Walsh SL, Geter-Douglas B, Strain EC, Bigelow GE (2001). Enadoline and butorphanol: evaluation of κ-agonists on cocaine pharmacodynamics and cocaine self-administration in humans. J Pharmacol Exp Ther 299: 147–158.
Winhusen TM, Kropp F, Lindblad R, Douaihy A, Haynes L, Hodgkins C et al (2014). Multisite, randomized, double-blind, placebo-controlled pilot clinical trial to evaluate the efficacy of buspirone as a relapse-prevention treatment for cocaine dependence. J Clin Psychiatry 75: 757–764.
Zayara AE, McIver G, Valdivia PN, Lominac KD, McCreary AC, Szumlinski KK (2011). Blockade of nucleus accumbens 5-HT2A and 5-HT2C receptors prevents the expression of cocaine-induced behavioral and neurochemical sensitization in rats. Psychopharmacology 213: 321–335.
Acknowledgements
We acknowledge the technical assistance of Jennifer Gough, Floyd Steele, and Kaycee Faunce. We also acknowledge Kevin Costa for writing the original version of the behavioral programs. Banks and Negus were responsible for study concept and design. Banks performed the data analysis. Banks and Negus drafted the manuscript. Both authors critically reviewed content and approved the final manuscript version for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Banks, M., Negus, S. Repeated 7-Day Treatment with the 5-HT2C Agonist Lorcaserin or the 5-HT2A Antagonist Pimavanserin Alone or in Combination Fails to Reduce Cocaine vs Food Choice in Male Rhesus Monkeys. Neuropsychopharmacol 42, 1082–1092 (2017). https://doi.org/10.1038/npp.2016.259
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2016.259
This article is cited by
-
Evaluation of the 5-HT2C receptor drugs RO 60-0175, WAY 161503 and mirtazepine in a preclinical model of comorbidity of depression and cocaine addiction
Pharmacological Reports (2023)
-
Novel Pharmacological Agents for the Treatment of Cocaine Use Disorder
Current Behavioral Neuroscience Reports (2022)
-
Improving Translational Research Outcomes for Opioid Use Disorder Treatments
Current Addiction Reports (2021)
-
Learning from lorcaserin: lessons from the negative clinical trial of lorcaserin to treat cocaine use disorder
Neuropsychopharmacology (2020)
-
Serotonin transporter protein in autopsied brain of chronic users of cocaine
Psychopharmacology (2020)