Abstract
Anxiety disorders are debilitating psychiatric illnesses with detrimental effects on human health. These heightened states of arousal are often in the absence of obvious threatening cues and are difficult to treat owing to a lack of understanding of the neural circuitry and cellular machinery mediating these conditions. Activation of noradrenergic circuitry in the basolateral amygdala is thought to have a role in stress, fear, and anxiety, and the specific cell and receptor types responsible is an active area of investigation. Here we take advantage of two novel cellular approaches to dissect the contributions of G-protein signaling in acute and social anxiety-like states. We used a chemogenetic approach utilizing the Gαs DREADD (rM3Ds) receptor and show that selective activation of generic Gαs signaling is sufficient to induce acute and social anxiety-like behavioral states in mice. Second, we use a recently characterized chimeric receptor composed of rhodopsin and the β2-adrenergic receptor (Opto-β2AR) with in vivo optogenetic techniques to selectively activate Gαs β-adrenergic signaling exclusively within excitatory neurons of the basolateral amygdala. We found that optogenetic induction of β-adrenergic signaling in the basolateral amygdala is sufficient to induce acute and social anxiety-like behavior. These findings support the conclusion that activation of Gαs signaling in the basolateral amygdala has a role in anxiety. These data also suggest that acute and social anxiety-like states may be mediated through signaling pathways identical to β-adrenergic receptors, thus providing support that inhibition of this system may be an effective anxiolytic therapy.
Similar content being viewed by others
INTRODUCTION
Anxiety is a fundamental behavioral response to environmental threats that prepares the body for immediate action by enhancing arousal. Baseline anxiety levels keep us alert and aware of our surroundings. However, anxiety as a psychiatric disease is characterized by a heightened state of arousal, often in the absence of an obvious threat (Lieb, 2005). Prolonged anxiety due to psychiatric disorders, traumatic life experience, genetic factors, or sustained stress is detrimental to physical and mental health.
Disorders such as: generalized anxiety disorder, panic disorder, phobias, obsessive compulsive disorder, and posttraumatic stress disorder often require tailored therapeutic intervention. Current therapies act via different mechanisms of action and target a myriad of circuits and systems. Benzodiazepines, SSRIs, atypical antipsychotics and β-blockers, and antagonists of β-adrenergic receptors (βARs), are all widely used clinically (Baker et al, 2011; Frishman and Saunders, 2011). Of these therapies, β-blockers have amassed a substantial body of work, particularly in peripheral systems, and are often used to treat social phobias (Schneier, 2011; Stein et al, 2004). However, there are very few basic neuropharmacological reports concerning βAR signaling within the central nervous system, specifically the basolateral amygdala, leading to debate as to whether the clinical efficacy of β-blockers as anxiolytics is due to receptor signaling peripherally, centrally or both.
Norepinephrine (NE) is a critical mediator of the stress ‘fight or flight’ response and acts through neural circuits that widely express β- and α-adrenergic receptors of which there are nine distinct subtypes (α1a,b,d, α2a,b,c β1–3) throughout the mammalian brain (Hieble et al, 1995). Release of NE is further modulated by the presence of presynaptic and postsynaptic α2 (Gαi) autoreceptors that inhibit NE release upon binding NE (Arima et al, 1998; Goddard et al, 2010; Grigg et al, 1996). Conversely, α1 (Gαq) and β1–3 (Gαs) adrenergic receptors are expressed postsynaptically causing mobilization of intracellular Ca2+ or cAMP, respectively. Each receptor type mediates disparate downstream effects and the interplay between them generates norepinephrine’s dynamic range in neural circuits.
Central NE is largely produced in two distinct brain regions, the locus coeruleus (LC) and A1/A2 cell groups in the brain stem. These populations send dense neuronal projections to the basolateral (BLA), central (CeA), and extended amygdala (in particular, the bed nucleus of the stria terminalis (BNST)) (Asan, 1998; Byrum and Guyenet, 1987; Woulfe et al, 1990; Zhang et al, 2013), regions known to be involved in affective behaviors (Berridge and Waterhouse, 2003; Davis, 1992; Valentino and Aston-Jones, 2010). These regions are also enriched in all the major subtypes of adrenergic receptors (Lein et al, 2007) making isolation of their respective contributions in the amygdaloid complex pharmacologically challenging. Anatomically speaking, this area is further complicated by the adjacent proximity of the BLA and CeA. The predominately glutamatergic (excitatory) BLA acts as a relay center, sending projections to the neighboring GABAergic (inhibitory) CeA, making the role of either region in anxiety-like behavior more difficult to dissect (Lüthi and Lüscher, 2014).
The basolateral nucleus of the amygdala (BLA) is one key region associated with anxiety behavior (Valentino et al, 1993), including humans (Feinstein et al, 2013). We know that, in rodents, βAR antagonists infused into the amygdala block fear memory enhancement following stressful stimuli (Al-Hasani et al, 2013; Mantsch et al, 2010; Quirarte et al, 1998; Schmidt and Weinshenker, 2014; Vranjkovic et al, 2014); is required for fear conditioning behavior (Cahill et al, 1994; Dębiec and Ledoux, 2004; Rogan et al, 1997); and systemic administration of the βAR antagonist propranolol reduces the spontaneous firing rate of neurons within the BLA (Buffalari and Grace, 2007; Ferry et al, 1997; Huang et al, 1996). Generally, β-adrenergic receptors have been linked to amygdala-dependent learning of negative affective memories, and a predominate focus of this receptor system has been examining its role in modulating fear-related behaviors (McGaugh, 1988, 2004; Strange and Dolan, 2004). Furthermore, activation of endogenous adrenergic tone from the LC has recently been shown to be both necessary and sufficient for stress-induced anxiety, and optogenetic-induction of LC-mediated anxiety-like behavior is sensitive to systemic blockade of β-adrenergic receptors (McCall et al, 2015). However, the putative regions of action for these effects or the signaling pathways utilized remain unknown.
To elucidate the roles of noradrenergic influence on BLA function and negative affective behavior, we utilized two novel approaches of examining receptor function in vivo. We first utilized chemogenetics to selectively control generic Gαs signaling in excitatory neurons of the BLA through the use of the Gαs DREADD rM3Ds (designer receptors exclusively activated by designer drugs) (Armbruster et al, 2007; Farrell et al, 2013; Guettier et al, 2009). Here we show that activation of generic Gαs signaling via within the BLA is sufficient to induce acute and social anxiety-like behavioral states. We then utilized a chimeric rhodopsin/β2-adrenergic receptor (Opto-β2AR; Airan et al, 2009) that has been recently demonstrated to mimic β2-adrenergic signaling both in vitro and in vivo (Siuda et al, 2015a). Using in vivo optogenetics, we selectively photostimulated β2AR signaling within the BLA and CeA and demonstrated that engagement of β2AR signaling selectively in the BLA, but not in the CeA, was sufficient to induce acute and social anxiety-like behavior in mice.
METHODS
Cell Culture
HEK293 cells were grown in Dulbecco’s modified Eagle's media supplemented with 10% fetal bovine serum containing 1 × pen/strep (Invitrogen) and maintained at 37 °C in a humidified incubator with 5% CO2. Plasmid containing Gαs DREADD was transfected into HEK293 cells using JetPrime (Polyplus) reagent per the manufacturer’s instructions.
Real-Time cAMP Assay
HEK293 cells were transfected with the pGloSensor-22F cAMP plasmid (Promega E2301) using JetPrime (Polyplus) transfection reagent per the manufacturer’s instructions. The day before an experiment, cells were plated in 96-well tissue culture-treated plates (Costar) at 25 000 cells/well and allowed to recover overnight at 37 °C, 5% CO2. The next day, media was replaced with 2% GloSensor reagent (Promega) suspended in CO2-independent growth medium (Gibco) and incubated for 2 h at 25 °C. For real-time cAMP, baseline relative luminescent units (RLUs) were recorded every 6 s for 1 min using a SynergyMx microplate reader (BioTek, Winooski, VT, USA). Clozapine-N-oxide (CNO; 10 μM; Sigma C0832) was then used to stimulate the cells, and subsequent RLUs were recorded every 6 s for 5–10 min at room temperature.
Animals
Adult (25–35 g) male C57BL/6J mice were group-housed, given access to food and water ad libitum, and maintained on a 12-h:12-h light:dark cycle. All animals were held in a sound attenuated, temperature controlled facility within the laboratory 1 week prior to surgery, postsurgery, and throughout the duration of the behavioral assays to minimize stress from transportation and disruption from foot traffic. All procedures were approved by the Animal Care and Use Committee of Washington University in St Louis and conformed to US National Institutes of Health guidelines.
Viral Preparation
Plasmids encoding pLenti-CaMKIIα-opto-β2AR-mCherry (final titer 4.8 × 108 IU/ml) and pAAV-hSyn-Optoβ2AR-eYFP (final titer 5 × 1012 vg/ml) were obtained from Deisseroth Laboratory at Stanford University and then packaged at the WUSTL Hope Center Viral Core. Lenti-PGK-GFP (final titer 1.3 × 108 IU/ml) and AAV-EF1α-YFP (final titer 5 × 1012 virus molecules/ml) were provided by the WUSTL viral core facility. AAV5-CaMKIIα-HA-GSD-IRES-mCitrine (final titer 3 × 1012 virus molecules/ml) and AAV5-CaMKIIα-eGFP (final titer 5 × 1012 virus molecules/ml) were obtained from University of North Carolina Gene Therapy Center Vector Core and Virus Vector Core. CaMKIIα and Synapsin promoters are used to selectively target excitatory neurons and pan-neuronal-specific expression, respectively (Zhang et al, 2010). Elongation factor 1α (EF1α) and phosphoglycerate kinase 1 (PGK) are constitutive promoters commonly used in mammalian systems (Qin et al, 2010).
Stereotaxic Surgery
Mice were anesthetized in an induction chamber (5% isoflurane) and placed in a stereotaxic frame (Kopf Instruments, Model 1900) where they were maintained at 1–2% isoflurane throughout the procedure. A craniotomy was performed, and mice were injected as follows. For basolateral amygdala injections, 1.2 μl of either lenti-PGK-GFP or lenti-CaMKIIα-optoβ2AR-mCherry or 0.5 μl of either AAV5-CaMKIIα-HA-GSD-IRES-mCitrine or AAV5-CaMKIIα-eGFP was injected bilaterally at stereotaxic coordinates from bregma: −1.3 mm (AP), ±2.9 mm (ML), and −4.9 mm (DV). For CeA injections, 1.0 μl of either AAV5-hSyn-opto-β2AR-eYFP or AAV5-EF1α-YFP was injected bilaterally at stereotaxic coordinates from bregma: −1.34 mm (AP), ±2.5 mm (ML), and −4.6 mm (DV). Mice were then implanted with chronic fiber optic implants with coordinates adjusted from viral injection to 0.00 mm (AP), ±0.25 mm (ML), and +1.00 mm (DV). The fiber optic implants were secured using two bone screws (CMA, 743102) and affixed with TitanBond (Horizon Dental Products) and dental cement (Lang Dental) (Al-Hasani et al, 2015; McCall et al, 2013). Mice were allowed to recover for at least 3 weeks prior to behavioral testing, allowing for optimal viral expression.
Behavior
Behavioral assays were performed in a sound attenuated room maintained at 23 °C. Lighting was measured and stabilized at ~4 lux for anxiety tests and ~200 lux for place testing. All behavioral apparatuses were cleaned with 70% ethanol in between animals. In photostimulation assays, mice with lenti-PGK-GFP, lenti-CaMKIIα-optoβ2AR-mCherry, AAV5-hSyn-opto-β2AR-eYFP or AAV5-EF1α-YFP received 5 s of constant photostimulation (473 nm, 1 W/cm2) followed by 5 s of no light (Airan et al, 2009; Siuda et al, 2015a) throughout the trial. In rM3Ds Gαs DREADD assays, mice with AAV5-CaMKIIα-HA-GSD-IRES-mCitrine and AAV5-CaMKIIα-eGFP received CNO (Sigma; 1 mg/kg, i.p.) in saline 30 min prior to behavioral testing. This dose of CNO is standard among users of these DREADD receptor types (Farrell et al, 2013; Ferguson et al, 2013; Guettier et al, 2009). All behavioral experiments were video recorded and analyzed using the Ethovision Software (Noldus v8.5). Behavioral outputs included time, distance, and velocity.
Behavior—Open Field Test (OFT)
As previously described (McCall et al, 2015), the open field was a 50 × 50 cm2 square plexiglass enclosure. Lighting was measured and stabilized at ~4 lux. For rM3Ds experiments, animals were dosed 1 mg/kg (i.p.) with CNO dissolved in saline 30 min prior to assay. For opto-β2AR experiments, animals were connected to fiber optic cables coupled to a master-9 function generator and placed in the center of the open field and allowed to roam freely for 30 min while receiving 473 nm blue light at 5 s on/off intervals at 1 W/cm2 power. The center was defined as a square comprising 50% the total area of the OFT. Mean time spent in the center was the primary measure of anxiety-like behavior.
Behavior—Social Approach (SA)
Social approach behavior was based and modified on previous studies (Silverman et al, 2010). Lighting was measured at ~200 lux. Mice were allowed to freely roam a two-chambered black, plastic box for 10 min that contained an inverted, metal, mesh pencil cup at each end. A novel, sex- and age-matched conspecific was then added to one of the inverted cups in a random, counterbalanced manner. The social zone is described as a circle that surrounds the inverted cup that is equal to twice its diameter. The test mouse was then reintroduced to the chamber and the time spent in the social zone recorded. For rM3Ds experiments, animals were dosed 1 mg/kg (i.p) with CNO dissolved in saline 30 min prior to the second phase of the assay. For opto-β2AR experiments, animals were connected to fiber optic cables coupled to a master-9 function generator and placed in the center of the chamber and allowed to roam freely for 10 min while receiving 473 nm blue light at 5 s on/off intervals at 1 W/cm2 power for the second phase of the assay.
Behavior—Conditioned Place Aversion (CPA)
Mice were trained in an unbiased, balanced three compartment conditioning apparatus as previously described (Al-Hasani et al, 2013; McCall et al, 2015). Lighting was measured at ~200 lux. Animals are placed in a three-chambered arena in which two chambers contain visually disparate contextual cues (horizontal or vertical stripes). Following an initial 30 min pretest, animals are conditioned over a 2-day period (conditioning day 1 (CD1) and conditioning day 2 (CD2)) with two training sessions per day (AM=without light, PM=with light) where the animal is confined to one chamber or the other. Each training session was separated by at least 4 h. Photostimulation (473 nm blue light at 5 s on/off intervals at 1 W/cm2 power) is randomly paired with one chamber, either horizontal or vertical, and the other chamber is paired with no photostimulation. On the fourth day, the animals are given access to all three chambers, and the amount of time spent in each chamber is recorded (posttest). Time spent in each compartment was recorded with a video camera (ZR90; Canon) and analyzed using Ethovision 8.5 (Noldus). CPA was assessed on day 4 by allowing the mice to roam freely in all three compartments and recording the time spent in each. Data are expressed as mean±SEM% time spent in the light stimulus-paired compartment.
Behavior—Real-Time Place Aversion (RTPA)
Mice were placed into a custom-made unbiased, balanced two-compartment conditioning apparatus (52.5 × 25.5 × 25.5 cm3) as previously described (Al-Hasani et al, 2015; McCall et al, 2015; Siuda et al, 2015b) and allowed to freely roam the entire apparatus for 20 min. Entry into one compartment triggered constant photostimulation (5 s on/off, 473 nm, 1 W/cm2 light power) while the animal remained in the light-paired chamber. Entry into the other chamber ended the photostimulation. The side paired with photostimulation was counterbalanced across mice. Time spent in each chamber and total distance traveled for the 20-min trial was measured using Ethovision 8.5 (Noldus). Data are expressed as mean±SEM% time spent in photostimulation-paired chamber.
Immunohistochemistry
After the conclusion of behavioral testing, mice were anesthetized with sodium pentobarbital and transcardially perfused with ice cold PBS, followed by 4% phosphate-buffered paraformaldehyde. Brains were removed, fixed overnight in paraformaldehyde, and saturated in 30% phosphate-buffered sucrose. In all, 30-μm sections were cut, washed in 0.3% Triton X100/5% normal goat serum in 0.1 M PBS, stained with fluorescent Nissl stain (1 : 400 Neurotrace, Invitrogen, Carlsbad, CA) for 1 h, and mounted onto glass slides with Vectashield (Vector Laboratories, Burlingame, CA). Viral expression was verified using fluorescence (Olympus, Center Valley, PA) and confocal microscopy (Leica Microsystems, Bannockburn, IL). Images were produced with the Leica Application Suite Advanced Fluorescence software. Animals that did not show targeted expression were excluded from analyses.
IHC was quantified as previously described (Al-Hasani et al, 2013; Kim et al, 2013). 1° Ab rabbit monoclonal α-CaMKIIα (EPR1828) (Abcam; ab92332; 1 : 500 in blocking buffer) and 2° Ab at 1 : 500 in PBS for opto-β2AR goat α rabbit IgG Alexa Fluor 488 and for GFP goat α rabbit IgG Alexa Fluor 594. Channels were separated, an exclusive threshold was set, and positive staining for each channel was counted in a blind-to-treatment manner using Metamorph. The counts from each channel were then overlaid, and the percentage of co-labeled cells were reported. For pLenti-CaMKIIα-opto-β2AR-mCherry, four sections from four different animals each were used to quantify the percentage of co-label. For Lenti-PGK-GFP, six sections from three different animals each were used to quantify co-label.
Statistics/Data Analysis
All data are expressed as mean±SEM. Statistical significance was taken as *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 as determined by Student’s t-test (paired and unpaired). For cumulative, nonlinear, time course data, multiple unpaired t-tests were performed. Statistical analyses were performed in GraphPad Prism 5.0.
RESULTS
Chemogenetic Activation of Gαs Signaling in the BLA is Sufficient to Induce Anxiogenic Behavior
Excitation of channelrhodopsin expressing BLA neurons has been previously shown to cause anxiogenic behavior (Felix-Ortiz et al, 2013; Tye et al, 2011), although the specific signaling pathways that mediate these effects are not known. Furthermore, in addition to numerous other G-protein coupled receptor (GPCR) types in the BLA that couple to diverse G-protein signaling modules, there are nine total adrenergic receptor types (α1AR–Gαq, α2AR–Gαi, and β1–3AR–Gαs). In addition, recent work suggested that β-adrenergic receptors mediate the anxiogenic effects following stimulation of locus coeruleus noradrenergic neurons (McCall et al, 2015). Thus we first used a global, yet region and cell-type selective approach to investigate the role of Gαs activation signaling within the BLA. We utilized DREADDS. These receptors are highly selective for coupling to Gαs pathways over other G-proteins (Ferguson et al, 2013; Guettier et al, 2009; Rogan and Roth, 2011). Utilizing the rM3Ds Gαs DREADD receptor packaged in a virus under a CAMKIIα promoter (rM3DsBLA/CaMKIIα), we bilaterally infused the virus or its control empty vector virus into the BLA and allowed at least 3 weeks for optimal viral expression (Figure 1a–c and Supplementary Figure S1a). Using the OFT, a common assay to investigate anxiety-like behavior in rodents, dosed mice with the DREADD-selective agonist CNO (1 mg/kg, i.p.) 30 min prior to testing (Figure 1c). rM3DsBLA/CaMKIIα animals (green; n=9) displayed a significant anxiogenic-like response throughout the 30-min OFT trial compared with control eGFP animals (n=12) (Figure 1d–f). This anxiety-like phenotype was evident within the first 10 min of the assay as demonstrated by a cumulative time course (Figure 1f; multiple unpaired Student’s t-tests; bin 1 t(19)=1.047, P=0.3081; bin 2 t(19)=2.881, P=0.0096; bin 3 t(19)=3.421, P=0.0029; bin 4 t(19)=3.689, P=0.0016; bin 5 t(19)=3.099, P=0.0059; bin 6 t(19)=3.084, P=0.0061). This anxiogenic-like behavior, however, had no effect on total distance traveled (Figure 1g unpaired Student’s t-test; t(18)=0.5084, P=0.6173) or velocity (Supplementary Figure S1b; unpaired Student’s t-test; t(18)=0.3499, P=0.7305), suggesting animal mobility was not affected. However, there was a reduction in the number of entries into the center of the open field suggesting that mice expressing rM3DsBLA/CaMKIIα made fewer entries into and spent less time in the center of the open field (Supplementary Figure S1c; unpaired Student’s t-test; t(18)=2.054, P=0.0548).
Gαs DREADD signaling in the BLA induces anxiety-like behavior. (a) Bilateral viral injection sites in the BLA. (b) AAV5-CaMKIIα-HA-GSD-IRES-mCitrine (rM3Ds; green) expression in BLA (DAPI=red; scale bar=50 μm). (c) Behavior diary. Representative open field traces of (d) control and (e) rM3DsBLA/CaMKIIα mice. (f) rM3DsBLA/CaMKIIα mice (green) show cumulatively less time in the center of the open field as compared with controls (black) following CNO administration (1 mg/kg, i.p.) (**P<0.01, multiple unpaired Student’s t-tests). (g) Distance traveled of rM3DsBLA/CaMKIIα and control mice.
Previous in vitro and in vivo studies using the rM3Ds receptor have suggested that this particular Gαs DREADD receptor is constitutively active (Guettier et al, 2009). To address this concern, we used a real-time cAMP assay to examine rM3Ds activity. HEK293 cells expressing the GloSensor plasmid (HEK-pGlo) were transfected with high levels of Gαs DREADD (Gαs-pGlo). Following an initial 1 min baseline, the Gαs-pGlo-expressing cells show a significant increase in cAMP following administration of CNO (10 μM) in stark contrast to empty HEK-pGlo cells (Supplementary Figure S1d). More importantly, if we expand the axes before and after addition of CNO (red box Supplementary Figure S1d), we see that the Gαs-pGlo cells maintain similar baseline cAMP values to HEK-pGlo, suggesting that there is no constitutive Gαs activity with high DREADD receptor expression in vitro (Supplementary Figure S1e and f; unpaired Student’s t-test; t(4)=0.8794, P=0.4288). Finally, if we examine the initial 5 min of the OFT data (Figure 1e), we see that there are no statistical differences between controls and Gαs-expressing animals (unpaired Student’s t-test; bin 1 t(19)=1.047, P=0.3081), suggesting again that there is no constitutive activity in vivo. Taken together, these data support the conclusion that activation of Gαs signaling within the BLA induces an acute anxiogenic state.
Chemogenetic Activation of Gαs Signaling in the BLA is Sufficient to Produce Social Anxiety-Like Behavior
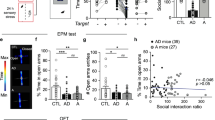
In addition to acute anxiety, we next examined the effects of Gαs activation in the BLA in a mouse behavioral model of social anxiety. Adapted from similar behavioral assays that model social anxiety-like states (Bailey and Crawley, 2009; Bruchas et al, 2011; Felix-Ortiz and Tye, 2014; Silverman et al, 2010), the social approach assay measures the time a mouse spends in the presence of a novel conspecific (social zone) (Figure 2a). Less time spent in the social zone indicates a social anxiety-like state that is reversible using traditional anxiolytics, such as diazepam (Stemmelin et al, 2007). We first demonstrated that animals expressing the empty vector eGFP control virus (n=15) or rM3DsBLA/CaMKIIα (n=11) spent similar amounts of time investigating the empty cup (Figure 2b and c, top panels and Figure 2d; unpaired Student’s t-test; t(24)=1.109, P=0.2785). Thirty minutes following CNO (1 mg/kg, i.p.) administration, control animals spent significantly more time in the social zone in the presence of a novel conspecific (Figure 2b, bottom panel and Figure 2d; paired Student’s t-test; t(14)=3.427, P=0.0041). In contrast, chemogenetic activation of Gαs signaling in the BLA following CNO administration induced a significant reduction in the time spent in the presence of a novel conspecific (Figure 2c, bottom panel and Figure 2d; paired Student’s t-test; t(10)=4.042, P=0.0024), suggesting that activation of Gαs-signaling in CamKIIα-positive BLA neurons is sufficient to promote social anxiety-like behavioral states. Furthermore, we found that, for both treatment groups, animals traveled significantly less total distance in the presence of a conspecific (Figure 2e; eGFP paired Student’s t-test; t(14)=6.882, P<0.0001 and rM3DsBLA/CaMKIIα paired Student’s t-test; t(10)=7.624, P<0.0001) with neither group significantly different than the other (eGFP vs rM3DsBLA/CaMKIIα without mouse unpaired Student’s t-test; t(24)=1.336, P=0.1939 and with mouse unpaired Student’s t-test; t(24)=0.8214, P=0.4195). Finally, rM3DsBLA/CaMKIIα animals made fewer entries into the social zone in the presence of a conspecific, suggesting that in addition to less time there were fewer entries into the social zone (Figure 2f; paired Student’s t-test; t(10)=4.312, P=0.0015). Control animals also trended to make fewer entries into the social zone consistent with animals spending more time with the novel conspecific, albeit this trend is not statistically significant (Figure 2f; paired Student’s t-test; t(14)=1.857, P=0.0844). Together, these data suggest that activation of Gαs signaling in excitatory neurons of the BLA is sufficient to promote acute and social anxiety-like states.
Gαs DREADD signaling in the BLA alters social interaction. (a) Behavior diary. Representative traces of (b) control and (c) rM3DsBLA/CaMKIIα mice in the absence (top) and presence (bottom) of a conspecific. (d) Control animals (black) spend more time in the social zone in the presence of a conspecific (solid bars) than in its absence (empty bars) (**P<0.01, paired Student’s t-test), while rM3DsBLA/CaMKIIα (green;) animals spend less time in the social zone in the presence of a conspecific (**P<0.01, paired Student’s t-test). (e) Both control (black) and rM3DsBLA/CaMKIIα (green) mice travel less distance in the presence of a novel mouse (solid bars) than in its absence (empty bars) (****P<0.0001, paired Student’s t-test). (f) Both control (black) and rM3DsBLA/CaMKIIα (green) mice travel less distance in the presence of a novel mouse (solid bars) than in its absence (empty bars) (****P<0.0001, paired Student’s t-test). (f) Control (black; P=0.084) and rM3DsBLA/CaMKIIα (green; **P<0.01 paired Student’s t-test) mice make fewer entries into the social zone in the presence of a novel mouse (solid bars) than in its absence (empty bars).
Photoactivation of β-Adrenergic Signaling In vivo Within the BLA Promotes Anxiety-Like Behavior
The rM3DsBLA/CaMKIIα data suggest that Gαs signaling within the BLA elicits anxiogenic behavioral phenotypes. Among the GPCRs present in the BLA, adrenergic receptors, and in particular β-adrenergic receptors (βARs), that signal via Gαs signaling are highly expressed (Lein et al, 2007). Previous reports have also shown that NE release in the BLA decreases neuronal firing, an effect predominately mediated via α2-adrenergic receptors (Gαi), whereas activation of βAR (Gαs) results in excitation (Buffalari and Grace, 2007; Davis et al, 1994; Menard and Treit, 1999). The central CeA, BLA and BNST are enriched in all the major subtypes of adrenergic receptors (Lein et al, 2007), making isolation of their respective contributions in the amygdaloid complex pharmacologically challenging. Thus, to gain better spatiotemporal isolation of adrenergic receptor pathways, we utilized the chimeric rhodopsin/β2-adrenergic receptor, opto-β2AR (Figure 3a). Opto-β2AR has been shown to activate cAMP, presumably through Gαs-mediated signaling, and to modulate neuronal excitability (Airan et al, 2009; Bailes et al, 2012; Kim et al, 2005; Siuda et al, 2015a), as such this chimeric receptor offers advantages over traditional pharmacological approaches in allowing cell-type specificity and greater spatiotemporal control over βAR signaling.
Photoactivation of Opto-β2AR in the BLA promotes anxiety-like behavior. (a) Bilateral viral injection sites and optic fiber implants. (b) Viral expression of lenti-CaMKIIα-opto-β2AR-mCherry in the BLA (scale bar=50 μm). (c) Behavior diary. Representative traces of control (d) and opto-β2ARBLA/CaMKIIα (e) mice in OFT. (f) Cumulative time course shows opto-β2ARBLA/CaMKIIα (orange) mice spend less time in the center of an open field compared with controls (black) while receiving light stimulation (473 nm, 5 s off/on; **P<0.01, via multiple unpaired Student’s t-tests). (g) In the OFT, viral control (black) and opto-β2ARBLA/CaMKIIα (orange) mice do not differ in total distance traveled.
We next explored whether selective activation of β-adrenergic signaling in BLA excitatory neurons using optogenetic approaches was sufficient to promote anxiety-like behavior. We first packaged opto-β2AR in a lentivirus under a CaMKIIα promoter and then bilaterally injected the virus (opto-β2ARBLA/CaMKIIα) or empty vector control virus into the BLA (Figure 3b). Following viral injection, we then implanted permanent optic ferrules slightly dorsal to the injection coordinates in the BLA (Figure 3c). We utilized the OFT as a model of acute anxiety-like behavior and photoactivated (473 nm, 5 s on/off, 1 W/cm2) animals via fiber optic cables throughout the assay. Photoactivation of opto-β2ARBLA/CaMKIIα (n=7) in the BLA produced rapid and sustained anxiogenic-like behavior with mice spending significantly less cumulative time in the center of the OFT than GFP controls (n=8) (Figure 3d–f). The anxiogenic effect was seen almost immediately and lasted throughout (Figure 3f; multiple unpaired Student’s t-tests; bin 1 t(13)=3.472, P=0.0041; bin 2 t(13)=3.367, P=0.0051; bin 3 t(13)=3.169, P=0.0074; bin 4 t(13)=3.725, P=0.0025; bin 5 t(13)=3.452, P=0.0043; bin 6 t(13)=3.463, P=0.0042). We observed no effect on animal mobility as both controls and opto-β2ARBLA/CaMKIIα animals showed no differences in total distance traveled (Figure 3h; unpaired Student’s t-test; t(13)=0.3221, P=0.7525) or velocity (Supplementary Figure S2a; unpaired Student’s t-test; t(13)=0.5324, P=0.6035). Interestingly there were no differences in number of entries into the center of the open field, suggesting that opto-β2ARBLA/CaMKIIα-expressing animals entered the open area but did not remain there for a long period of time (Supplementary Figure S2b; unpaired Student’s t-test; t(13)=0.7522, P=0.4653).
These data suggest that activation of β-adrenergic signaling in BLA excitatory neurons is anxiogenic. Given that the BLA is composed predominately of excitatory neurons (Carlsen, 1988; Smith and Paré, 1994), we believe that a CaMKIIα promoter should label the vast majority of neurons within the BLA. To verify this, viral expression of lenti-CaMKIIα-opto-β2AR-mCherry and lenti-PGK-GFP was confirmed in a subset of animals and stained for CaMKIIα. Approximately 96% of lenti-opto-β2AR-mCherry+ cells and lenti-PGK-GFP co-labeled with CaMKIIα+ cells in the BLA suggesting expression driven under the CaMKIIα promoter was efficient (Supplementary Figure S2c and d; unpaired Student’s t-test; t(8)=0.02041, P=0.9842). These data corroborate others using CaMKIIα co-labeling in the BLA (Johansen et al, 2010). Similar results were obtained with lenti-PGK-GFP+ cells as PGK is a constitutive and fairly ubiquitous promoter (Qin et al, 2010).
To test for regional specificity of viral expression, photo-illumination, and functional isolation of this β-adrenergic signaling response, we also examined the effects of opto-β2AR stimulation when expressed in neurons of the CeA (opto-β2ARCeA/hSyn) (Supplementary Figure S3a and b). As the cytoarchitecture of the CeA is more heterogeneous than the BLA and is predominately GABAergic (Lüthi and Lüscher, 2014), opto-β2AR was packaged under the pan-neuronal human synapsin promoter as previously used in the CeA for optogenetic studies (Robinson et al, 2014). Using the same experimental paradigm (Supplementary Figure S3c), photostimulation (473 nm, 5 s on/off, 1 W/cm2) of opto-β2ARCeA/hSyn (n=6) did not result in an anxiogenic phenotype (Supplementary Figure S3d–f; multiple unpaired Student’s t-tests; bin 1 t(10)=0.1040, P=0.9192; bin 2 t(10)=0.09359, P=0.9273; bin 3 t(10)=0.05840, P=0.9546; bin 4 t(10)=0.05844, P=0.9546; bin 5 t(10)=0.08324, P=0.9353; bin 6 t(10)=0.01982, P=0.9846). and had no effect on animal mobility (Supplementary Figure S3g–i; distance t(9)=1.771, P=0.1103; velocity t(8)=1.717, P=0.1243; entries t(10)=0.6925, P=0.5044) in comparison to YFP controls (n=6). These data suggest that β-adrenergic signaling in the BLA, but not in the CeA, is important in acute anxiety-like states.
Photoactivation of β-Adrenergic Signaling Within the BLA Does not Promote Real-Time or Conditioned Aversion
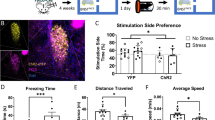
Previous studies have shown that activation of LC cell bodies via channelrhodopsin induces an aversive behavioral phenotype in a RTPA assay, which was reversed with antagonism at α1-adrenergic receptors (prazosin) but not β-adrenergic (propranolol) (McCall et al, 2015). However, anxiogenic behavior induced by tonic optogenetic activation of the LC was indeed sensitive to systemic β-adrenergic receptor blockade. We used two models of assessing aversion in mice: RTPA and CPA (Al-Hasani et al, 2015; McCall et al, 2015). Opto-β2ARBLA/CaMKIIα- (n=7) and viral GFP control-expressing mice (n=5) were photostimulated (473 nm, 5 sec on/off, 1 W/cm2) in real time when entering a chamber randomly paired to photostimulation (Figure 4a and b). Photostimulation was terminated when the animal left the chamber. Consistent with recent reports suggesting that β-adrenergic receptors do not mediate noradrenergic-dependent real-time aversion (McCall et al, 2015), during this 20-min trial opto-β2ARBLA/CaMKIIα- and viral control-expressing mice showed no differences in the amount of time spent in the photostimulation-paired chamber (Figure 4c; unpaired Student’s t-test; t(10)=0.9059, P=0.3863) or in general mobility (Figure 4d; unpaired Student’s t-test; t(10)=0.5893, P=0.5687). These data suggest that activation of βAR signaling within excitatory neurons of the BLA does not induce an acute aversive state.
Photoactivation of Opto-β2AR does not alter RPTA or CPA. (a) Behavior diary and (b) chamber schematic. (c) Opto-β2ARBLA/CaMKIIα (orange) and control mice (black) spend similar amounts of time in the photostimulation-paired chamber in real-time place aversion (RTPA) and show no differences in total distance traveled (d). (e) Behavior diary and (f) chamber schematic. (g) Opto-β2ARBLA/CaMKIIα (orange) do not show an aversive response to the condition-photostimulation chamber. (h) Opto-β2ARBLA/CaMKIIα (orange) and control animals (black) show no differences in distances traveled during conditioning day 1 (CD1) and or conditioning day 2 (CD2) in CPA.
Previous studies have also shown that β-adrenergic activity within the amygdala has a key role in fear memory and fear conditioning, both of which possess a learning component (Cahill et al, 1994; Dębiec and Ledoux, 2004; Quirarte et al, 1998; Rogan et al, 1997). To determine whether βAR activation in the BLA alters aversive learning, we photostimulated opto-β2ARBLA/CaMKIIα during the CPA assay (Figure 4e and f). Opto-β2ARBLA/CaMKIIα-expressing mice (n=7) showed no difference in the amount of time spent in the photostimulation-paired chamber following 2 days of conditioning (Figure 4g; paired Student’s t-test; t(6)=0.4447, P=0.6721) and showed no differences in mobility compared with empty vector viral GFP controls (Figure 4h; two-way ANOVA for repeated measurements, time F(5,25)=6.10, P=0.0008, virus F(1,5)=3.88, P=0.1060, interaction F(5,25)=1.16, P=0.3560). These data suggest that activation of βAR signaling within excitatory neurons of the BLA does not effect conditioned aversion. In summary, these data suggest that activation of βAR signaling within the BLA has effects on acute anxiety-like behavior but not in either acute or conditioned aversive states.
Optogenetic Activation of Opto-β2AR Signaling Within the BLA is Sufficient to Induce Social Anxiety-Like Behavior
β-Adrenergic receptors have also been implicated in social anxiety disorders (Liebowitz, 1987) and β-blockers are commonly prescribed as anxiolytics clinically (Baker et al, 2011). Having shown that activation of generic Gαs signaling within the BLA disrupted social behavior via DREADD receptors (Figure 2d), and knowing prior studies have shown that the BLA is critical for these behaviors (Felix-Ortiz and Tye, 2014), we posited that activation of βAR signaling would have similar effects. Utilizing the social approach assay (Figure 5a), we show that both opto-β2ARBLA/CaMKIIα- (n=8) and viral control GFP-expressing mice (n=7) spend similar amounts of time in the social zone in the absence of a novel conspecific (Figure 5b and c, top panels and Figure 5d; unpaired Student’s t-test; t(13)=0.7423, P=0.4711). However, in the presence of a novel conspecific and while receiving photostimulation, GFP control animals showed a significant increase in the time spent in the social zone (Figure 5b, bottom panel and Figure 5d; paired Student’s t-test; t(6)=3.567, P=0.0118), whereas opto-β2ARBLA/CaMKIIα animals did not display this social interaction behavior (Figure 5c, bottom panel and Figure 5d; paired Student’s t-test; t(7)=0.4419, P=0.6719). Both groups showed a significant reduction in the total distance traveled in the presence of the novel conspecific (Figure 5e; GFP paired Student’s t-test; t(6)=3.253, P=0.0174 and opto-β2ARBLA/CaMKIIα paired Student’s t-test; t(7)=10.26, P<0.0001) with neither group significantly different than the other (GFP vs opto-β2ARBLA/CaMKIIα without mouse unpaired Student’s t-test; t(13)=0.5590, P=0.5857 and with mouse unpaired Student’s t-test; t(13)=1.238, P=0.2376). Both groups also had similar entries into the social zone (Figure 5f; GFP paired Student’s t-test; t(6)=0.7285, P=0.4937 and opto-β2ARBLA/CaMKIIα paired Student’s t-test; t(6)=2.213, P=0.0689). These data suggest that activation of opto-β2AR signaling in the BLA is sufficient to induce acute social anxiety-like states.
Photoactivation of Opto-β2AR in the BLA alters social behavior. (a) Behavior diary. Representative traces of (b) control and (c) opto-β2ARBLA/CaMKIIα in the absence (top) and presence (bottom) of a novel conspecific. (d) Control animals (black) spend more time in the social zone in the presence of a novel conspecific (solid bars) than in its absence (empty bars) (*P<0.05, paired Student’s t-test), while opto-β2ARBLA/CaMKIIα (orange) animals show no increased time spent in the social zone in the presence of a conspecific. (e) Both control (black; *P<0.05 paired Student’s t-test) and opto-β2ARBLA/CaMKIIα (orange; ****P<0.0001, paired Student’s t-test) mice travel less distance in the presence of a novel mouse (solid bars) than in its absence (empty bars). (f) Both control (black) and opto-β2ARBLA/CaMKIIα (orange) animals show similar number of entries into the social zone in the presence (solid bars) or absence (empty bars) of a novel mouse.
Taken together, these data demonstrate that in vivo photoactivation of opto-β2ARBLA/CaMKIIα in the BLA induces an anxiety-like behavioral phenotype. We also report that this effect is selective to the BLA, as animals transduced with opto-β2AR in the neighboring CeA showed no acute anxiety-like behavior. In addition, we found that social interaction time in photoactivated opto-β2ARBLA/CaMKIIα animals is diminished in comparison to controls, suggesting an important role of this Gαs-signaling pathway in mediating social behavior.
DISCUSSION
The BLA is one key region particularly associated with anxiety and mood (Feinstein et al, 2013; Valentino et al, 1993). The BLA receives noradrenergic input from the LC (Asan, 1998; Fallon et al, 1978) and sends projections to the CeA, the prefrontal cortex, nucleus accumbens, and BNST, regions linked to mood regulation, and some with very disparate effects (Tye and Deisseroth, 2012). For example, in vivo photostimulation of BNST glutamatergic projections is both aversive and anxiogenic (Jennings et al, 2013), while activation of BLA terminals specifically in the CeA is anxiolytic (Tye et al, 2011). Thus teasing out the relative contributions of each region has been historically challenging with traditional pharmacological techniques.
The advent of chemogenetic and optogenetic techniques has enabled the selective targeting and manipulation of specific cell types and brain regions (Namburi et al, 2015). Previous studies have shown that photostimulation of CaMKIIα+ channelrhodopsin-expressing cell bodies in the BLA produced an anxiogenic phenotype (Tye et al, 2011). Although highlighting the important role of the BLA in anxiogenesis, the use of channelrhodopsin itself limits the unraveling of potential neuromodulatory mechanisms of action. In the present study, we utilized chemogenetic and optogenetic approaches to manipulate G-protein coupled cellular activity through more endogenous neuromodulatory mechanisms known to regulate the gain of these neural circuits.
The rM3Ds DREADD receptor mimics generic Gαs intracellular signaling (Guettier et al, 2009). The BLA endogenously expresses a host of G-protein coupled receptors, many via Gαs mechanisms (Lein et al, 2007). Here we show that, when localized to CaMKIIα+ neurons of the BLA, systemic administration of the DREADD agonist CNO produces not just acute anxiety but also induces deficits in social interaction between conspecifics. These results corroborate previous studies that photostimulated channelrhodopsin-expressing BLA terminals in the ventral hippocampus, which produced reduced social behaviors in the resident-juvenile intruder procedure and decreased time in the social zone in the three chamber sociability test (Felix-Ortiz et al, 2013). While in agreement with other studies, our results are unique in that they dissect the cellular signaling mechanisms and are less binary (on/off) than the previous studies using channelrhodopsin. However, Gαs signaling via chemogenetic approaches comes with some limitations. While still activating intracellular cAMP, Gαs DREADDs act as a ‘generic’ Gαs GPCR, it is currently unclear whether this tool exhibits identical intracellular signaling cascades comparable to the endogenous Gαs receptors expressed within the BLA. We now know, for instance, that all pools of Gαs are not identical and that receptors signal to G-proteins in a very selective microdomain-dependent manner that is limited by receptor subtype (Irannejad et al, 2013; Puthenveedu et al, 2010). Studies thoroughly comparing the pharmacodynamic properties of Gαs DREADDs to canonical GPCRs, such as β2AR, are needed to provide further validation. Chemogenetic approaches are also faced with similar pharmacokinetic and pharmacodynamic issues as more traditional pharmacological approaches, thus highlighting the need to complement multiple techniques and alternative approaches to fully examine the signaling and neurocircuitry mediating affective behavioral states. In this case, the use of chemogenetic technology narrowed the window of receptor systems involved to Gαs signaling, likely to βAR signaling pathways. However, we do not rule out the possible contribution of other Gαs GPCRs expressed in the BLA and future studies utilizing other DREADD receptors, such as Gαi and Gαq, would complement our findings and further narrow down potential targets mediating acute and social anxiety-like states.
Manipulation of endogenous intracellular signaling in vivo has historically required pharmacological techniques. The use of optically active rhodopsin chimeric receptors, however, has allowed us to manipulate neurocircuitry in vivo, with higher specificity to endogenous mechanisms (Airan et al, 2009). Currently, there are several chimeric receptors that upon photoactivation are able to signal intracellularly via G-protein- and arrestin-mediated cascades: α1-adrenergic, β2-adrenergic, adenosine A2A, 5HT1A, μ-opioid receptor, and the D1 dopamine receptor (Airan et al, 2009; Bailes et al, 2012; Barish et al, 2013; Franke et al, 1992; Gunaydin et al, 2014; Kim et al, 2005; Li et al, 2015; Oh et al, 2010; Siuda et al, 2015a, b). Here we incorporate the chimeric rhodopsin/β2-adrenergic receptor (opto-β2AR) and show that activation of CaMKIIα+ neurons in the BLA via simulated β-adrenergic activation induces both acute and social anxiety-like states. These findings are in agreement with our chemogenetic data presented here and the recent channelrhodopsin data from other groups (Felix-Ortiz et al, 2013; Tye et al, 2011). Our stimulation paradigm (5 s on/off) was based on previous studies that resulted in a robust behavioral phenotype (Siuda et al, 2015a). Other studies with this chimeric receptor used a 10-Hz (50 ms) stimulation paradigm that resulted in no significant behavioral output in the nucleus accumbens, a region with low β-adrenergic receptor expression (Airan et al, 2009). We know that NE release in the BLA is predicated on inputs from the LC, whose activity contributes to an animal’s arousal state (Aston-Jones and Cohen, 2005; Berridge and Waterhouse, 2003; Bouret and Sara, 2005; Chang and Grace, 2013). At rest, the LC is spontaneously active, while acute stress shifts the firing patterns to increased tonic activity (5–8 Hz) or initiates phasic bursting to cause temporally distinct release of NE (Abercrombie and Jacobs, 1987a, b; Galvez et al, 1996; Mana and Grace, 1997; Quirarte et al, 1998). The kinetics of NE release, degradation, and uptake have been loosely examined (Iversen, 1971; Pelton et al, 1981), and as such it is unclear whether our stimulation paradigm truly mimics endogenous NE release. Thus it is possible that utilizing a different stimulation paradigm could result in different behavioral outputs and could represent different elements of noradrenergic tone into the BLA.
While anxiogenic when expressed in the BLA, opto-β2AR stimulation had no effect when expressed in neurons of the CeA. Others have shown that stimulating channelrhodopsin-expressing BLA terminals in the CeA produced an anxiolytic effect (Tye et al, 2011). The CeA is known to mediate conditioned fear and the acquisition of fear conditioning via inhibitory circuitry (Ciocchi et al, 2010; Haubensak et al, 2010; Wilensky et al, 2006). It is possible that ubiquitous expression of our construct was not sufficient to yield an obvious behavioral phenotype and may have affected the microcircuitry within the CeA itself.
Previous studies show that activation of the lateral amygdala is involved in fear learning (Johansen et al, 2010). Here we show that activating opto-β2AR in the CaMKIIα+ neurons of the BLA had no effect on CPA or RTPA. Although consistent with recent reports suggesting that β-adrenergic receptors do not mediate noradrenergic dependent real-time aversion (McCall et al, 2015), it is somewhat surprising that there was no effect in the CPA assay. Given the role of the BLA and β-adrenergic receptors in emotional and stressful memory (Bernardi et al, 2009; McGaugh, 2004; Wu et al, 2014), it is possible that our stimulus was not aversive or salient enough. Future studies could expand on the photostimulation parameters, and other learning assays, such as fear conditioning, may be more suited.
Our results suggest that noradrenergic influence in the BLA is mediated via activation of neuromodulatory β-adrenergic Gαs-signaling pathways that may ultimately promote both acute and social anxiogenic-like behavioral states. Although these data were collected from male mice only, recent evidence suggests structural sexual dimorphisms of the LC and sensitivity of female LC neurons may contribute to higher susceptibility rates to mood and anxiety disorders in females (Bangasser and Valentino, 2014; Bangasser et al, 2015). We can only speculate that our data can be extrapolated to females, and we believe this is an important area for future investigation.
In summary, we show here a role of Gαs signaling within the BLA in mediating acute and social anxiety-like behavioral states. These results suggest that noradrenergic influence on signaling into the BLA may have important consequences for generating anxiogenic behaviors; however, further studies of these receptors, circuits, and pathways are required. These results provide new insights into the receptors, cells, and circuits that mediate anxiety-like behavior and extend our understanding of the development of therapeutics for treating anxiety and stress disorders.
FUNDING AND DISCLOSURE
This work is supported by NIDA R01DA037152 (to MRB), R21DA035144 (to MRB), R00DA025182 (to MRB), NIMH F31MH101956 (to JGM), TR01NS081707, and the McDonnell Center for Systems Neuroscience (to MRB). The authors declare no conflict of interest.
References
Abercrombie ED, Jacobs BL (1987a). Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. I. Acutely presented stressful and nonstressful stimuli. J Neurosci 7: 2837–2843.
Abercrombie ED, Jacobs BL (1987b). Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. II. Adaptation to chronically presented stressful stimuli. J Neurosci 7: 2844–2848.
Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K (2009). Temporally precise in vivo control of intracellular signalling. Nature 458: 1025–1029.
Al-Hasani R, McCall JG, Foshage AM, Bruchas MR (2013). Locus coeruleus kappa-opioid receptors modulate reinstatement of cocaine place preference through a noradrenergic mechanism. Neuropsychopharmacology 38: 2484–2497.
Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM et al (2015). Distinct subpopulations of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron 87: 1063–1077.
Arima J, Kubo C, Ishibashi H, Akaike N (1998). alpha2-Adrenoceptor-mediated potassium currents in acutely dissociated rat locus coeruleus neurones. J Physiol 508 Pt 1: 57–66.
Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL (2007). Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci 104: 5163–5168.
Asan E (1998). The catecholaminergic innervation of the rat amygdala. Adv Anat Embryol Cell Biol 142: 1–118.
Aston-Jones G, Cohen JD (2005). An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28: 403–450.
Bailes HJ, Zhuang L-Y, Lucas RJ (2012). Reproducible and sustained regulation of gαs signalling using a metazoan opsin as an optogenetic tool. PLoS One 7: e30774.
Bailey KR, Crawley JN Anxiety-Related Behaviors in Mice. In: Buccafusco JJ (ed). Methods of Behavioral Analysis in Neuroscience. 2nd edn. Chapter 5. CRC Press: Boca Raton (FL); (2009).
Baker JG, Hill SJ, Summers RJ (2011). Evolution of β-blockers: from anti-anginal drugs to ligand-directed signalling. Trends Pharmacol Sci 32: 227–234.
Bangasser DA, Valentino RJ (2014). Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol 35: 303–319.
Bangasser DA, Wiersielis KR, Khantsis SM (2015). Sex differences in the locus coeruleus-norepinephrine system and its regulation by stress. Brain Res (doi:10.1016/j.brainres.2015.11.021).
Barish PA, Xu Y, Li J, Sun J, Jarajapu YPR, Ogle WO (2013). Design and functional evaluation of an optically active μ-opioid receptor. Eur J Pharmacol 705: 42–48.
Bernardi RE, Ryabinin AE, Berger SP, Lattal KM (2009). Post-retrieval disruption of a cocaine conditioned place preference by systemic and intrabasolateral amygdala beta2- and alpha1-adrenergic antagonists. Learn Mem 16: 777–789.
Berridge CW, Waterhouse BD (2003). The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42: 33–84.
Bouret S, Sara SJ (2005). Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci 28: 574–582.
Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB et al (2011). Selective p38α MAPK deletion in serotonergic neurons produces stress resilience in models of depression and addiction. Neuron 71: 498–511.
Buffalari DM, Grace AA (2007). Noradrenergic modulation of basolateral amygdala neuronal activity: opposing influences of α-2 and β receptor activation. J Neurosci 27: 12358–12366.
Byrum CE, Guyenet PG (1987). Afferent and efferent connections of the A5 noradrenergic cell group in the rat. J Comp Neurol 261: 529–542.
Cahill L, Prins B, Weber M, McGaugh JL (1994). β-Adrenergic activation and memory for emotional events. Nature 371: 702–704.
Carlsen J (1988). Immunocytochemical localization of glutamate decarboxylase in the rat basolateral amygdaloid nucleus, with special reference to GABAergic innervation of amygdalostriatal projection neurons. J Comp Neurol 273: 513–526.
Chang C, Grace AA (2013). Amygdala β-noradrenergic receptors modulate delayed downregulation of dopamine activity following restraint. J Neurosci 33: 1441–1450.
Ciocchi S, Herry C, Grenier F, Wolff SBE, Letzkus JJ, Vlachos I et al (2010). Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468: 277–282.
Davis M (1992). The role of the amygdala in fear and anxiety. Annu Rev Neurosci 15: 353–375.
Davis M, Rainnie D, Cassell M (1994). Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci 17: 208–214.
Dębiec J, Ledoux JE (2004). Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience 129: 267–272.
Fallon JH, Koziell DA, Moore RY (1978). Catecholamine innervation of the basal forebrain II. Amygdala, suprarhinal cortex and entorhinal cortex. J Comp Neurol 180: 509–531.
Farrell MS, Pei Y, Wan Y, Yadav PN, Daigle TL, Urban DJ et al (2013). A Gαs DREADD mouse for selective modulation of cAMP production in striatopallidal neurons. Neuropsychopharmacology 38: 854–862.
Feinstein JS, Buzza C, Hurlemann R, Follmer RL, Dahdaleh NS, Coryell WH et al (2013). Fear and panic in humans with bilateral amygdala damage. Nat Neurosci 16: 270–272.
Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, Tye KM (2013). BLA to vHPC inputs modulate anxiety-related behaviors. Neuron 79: 658–664.
Felix-Ortiz AC, Tye KM (2014). Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J Neurosci 34: 586–595.
Ferguson SM, Phillips PEM, Roth BL, Wess J, Neumaier JF (2013). Direct-pathway striatal neurons regulate the retention of decision-making strategies. J Neurosci 33: 11668–11676.
Ferry B, Magistretti PJ, Pralong E (1997). Noradrenaline modulates glutamate-mediated neurotransmission in the rat basolateral amygdala in vitro. Eur J Neurosci 9: 1356–1364.
Franke RR, Sakmar TP, Graham RM, Khorana HG (1992). Structure and function in rhodopsin. Studies of the interaction between the rhodopsin cytoplasmic domain and transducin. J Biol Chem 267: 14767–14774.
Frishman WH, Saunders E (2011). β-Adrenergic blockers. J Clin Hypertens 13: 649–653.
Galvez R, Mesches MH, Mcgaugh JL (1996). Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiol Learn Mem 66: 253–257.
Goddard AW, Ball SG, Martinez J, Robinson MJ, Yang CR, Russell JM et al (2010). Current perspectives of the roles of the central norepinephrine system in anxiety and depression. Depress Anxiety 27: 339–350.
Grigg JJ, Kozasa T, Nakajima Y, Nakajima S (1996). Single-channel properties of a G-protein-coupled inward rectifier potassium channel in brain neurons. J Neurophysiol 75: 318–328.
Guettier J-M, Gautam D, Scarselli M, Ruiz de Azua I, Li JH, Rosemond E et al (2009). A chemical-genetic approach to study G protein regulation of beta cell function in vivo. Proc Natl Acad Sci USA 106: 19197–19202.
Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A et al (2014). Natural neural projection dynamics underlying social behavior. Cell 157: 1535–1551.
Haubensak W, Kunwar PS, Cai H, Ciocchi S, Wall NR, Ponnusamy R et al (2010). Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468: 270–276.
Hieble JP, Bylund DB, Clarke DE, Eikenburg DC, Langer SZ, Lefkowitz RJ et al (1995). International union of pharmacology. X. Recommendation for nomenclature of alpha 1-adrenoceptors: consensus update. Pharmacol Rev 47: 267–270.
Huang CC, Hsu KS, Gean PW (1996). Isoproterenol potentiates synaptic transmission primarily by enhancing presynaptic calcium influx via P- and/or Q-type calcium channels in the rat amygdala. J Neurosci 16: 1026–1033.
Irannejad R, Tomshine JC, Tomshine JR, Chevalier M, Mahoney JP, Steyaert J et al (2013). Conformational biosensors reveal GPCR signalling from endosomes. Nature 495: 534–538.
Iversen LL (1971). Role of transmitter uptake mechanisms in synaptic neurotransmission. Br J Pharmacol 41: 571–591.
Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL et al (2013). Distinct extended amygdala circuits for divergent motivational states. Nature 496: 224–228.
Johansen JP, Hamanaka H, Monfils MH, Behnia R, Deisseroth K, Blair HT et al (2010). Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc Natl Acad Sci USA 107: 12692–12697.
Kim J-M, Hwa J, Garriga P, Reeves PJ, RajBhandary UL, Khorana HG (2005). Light-driven activation of β2-adrenergic receptor signaling by a chimeric rhodopsin containing the β2-adrenergic receptor cytoplasmic loops. Biochemistry 44: 2284–2292.
Kim T, McCall JG, Jung YH, Huang X, Siuda ER, Li Y et al (2013). Injectable, cellular-scale optoelectronics with applications for wireless optogenetics. Science 340: 211–216.
Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A et al (2007). Genome-wide atlas of gene expression in the adult mouse brain. Nature 445: 168–176.
Li P, Rial D, Canas PM, Yoo J-H, Li W, Zhou X et al (2015). Optogenetic activation of intracellular adenosine A2A receptor signaling in the hippocampus is sufficient to trigger CREB phosphorylation and impair memory. Mol Psychiatry 20: 1481.
Lieb R (2005). Anxiety disorders: clinical presentation and epidemiology. Handb Exp Pharmacol 405–432.
Liebowitz MR (1987). Social phobia. Mod Probl Pharmacopsychiatry 22: 141–173.
Lüthi A, Lüscher C (2014). Pathological circuit function underlying addiction and anxiety disorders. Nat Neurosci 17: 1635–1643.
Mana MJ, Grace AA (1997). Chronic cold stress alters the basal and evoked electrophysiological activity of rat locus coeruleus neurons. Neuroscience 81: 1055–1064.
Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H (2010). Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for β-2 adrenergic receptors. Neuropsychopharmacology 35: 2165–2178.
McCall JG, Al-Hasani R, Siuda ER, Hong DY, Norris AJ, Ford CP et al (2015). CRH engagement of the locus coeruleus noradrenergic system mediates stress-induced anxiety. Neuron 87: 605–620.
McCall JG, Kim T, Shin G, Huang X, Jung YH, Al-Hasani R et al (2013). Fabrication and application of flexible, multimodal light-emitting devices for wireless optogenetics. Nat Protoc 8: 2413–2428.
McGaugh JL (2004). The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 27: 1–28.
McGaugh JL, Introini-Collison IB, Nagahara AH (1988). Memory-enhancing effects of posttraining naloxone: involvement of beta-noradrenergic influences in the amygdaloid complex. Brain Res 446: 37–49.
Menard J, Treit D (1999). Effects of centrally administered anxiolytic compounds in animal models of anxiety. Neurosci Biobehav Rev 23: 591–613.
Namburi P, Al-Hasani R, Calhoon GG, Bruchas MR, Tye KM (2015). Architectural representation of valence in the limbic system. Neuropsychopharmacology (doi:10.1038/npp.2015.358).
Oh E, Maejima T, Liu C, Deneris E, Herlitze S (2010). Substitution of 5-HT1A receptor signaling by a light-activated G protein-coupled receptor. J Biol Chem 285: 30825–30836.
Pelton EW II, Kimelberg HK, Shipherd SV, Bourke RS (1981). Dopamine and norepinephrine uptake and metabolism by astroglial cells in culture. Life Sci 28: 1655–1663.
Puthenveedu MA, Lauffer B, Temkin P, Vistein R, Carlton P, Thorn K et al (2010). Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell 143: 761–773.
Qin JY, Zhang L, Clift KL, Hulur I, Xiang AP, Ren B-Z et al (2010). Systematic comparison of constitutive promoters and the doxycycline-inducible promoter. PLoS One 5: e10611.
Quirarte GL, Galvez R, Roozendaal B, McGaugh JL (1998). Norepinephrine release in the amygdala in response to footshock and opioid peptidergic drugs. Brain Res 808: 134–140.
Robinson MJF, Warlow SM, Berridge KC (2014). Optogenetic excitation of central amygdala amplifies and narrows incentive motivation to pursue one reward above another. J Neurosci 34: 16567–16580.
Rogan SC, Roth BL (2011). Remote control of neuronal signaling. Pharmacol Rev 63: 291–315.
Rogan MT, Stäubli UV, LeDoux JE (1997). Fear conditioning induces associative long-term potentiation in the amygdala. Nature 390: 604–607.
Schmidt KT, Weinshenker D (2014). Adrenaline rush: the role of adrenergic receptors in stimulant-induced behaviors. Mol Pharmacol 85: 640–650.
Schneier FR (2011). Pharmacotherapy of social anxiety disorder. Expert Opin Pharmacother 12: 615–625.
Silverman JL, Yang M, Lord C, Crawley JN (2010). Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci 11: 490–502.
Siuda ER, Copits BA, Schmidt MJ, Baird MA, Al-Hasani R, Planer WJ et al (2015b). Spatiotemporal control of opioid signaling and behavior. Neuron 86: 923–935.
Siuda ER, McCall JG, Al-Hasani R, Shin G, Park S II, Schmidt MJ et al (2015a) Optodynamic simulation of β-adrenergic receptor signalling. Nat Commun 6: 8480.
Smith Y, Paré D (1994). Intra-amygdaloid projections of the lateral nucleus in the cat: PHA-L anterograde labeling combined with postembedding GABA and glutamate immunocytochemistry. J Comp Neurol 342: 232–248.
Stein DJ, Ipser JC, Balkom AJ (2004) Pharmacotherapy for social phobia. Cochrane Database Syst Rev CD001206.
Stemmelin J, Cohen C, Terranova J-P, Lopez-Grancha M, Pichat P, Bergis O et al (2007). Stimulation of the β3-adrenoceptor as a novel treatment strategy for anxiety and depressive disorders. Neuropsychopharmacology 33: 574–587.
Strange BA, Dolan RJ (2004). β-Adrenergic modulation of emotional memory-evoked human amygdala and hippocampal responses. Proc Natl Acad Sci USA 101: 11454–11458.
Tye KM, Deisseroth K (2012). Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci 13: 251–266.
Tye KM, Prakash R, Kim S-Y, Fenno LE, Grosenick L, Zarabi H et al (2011). Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471: 358–362.
Valentino R, Aston-Jones G (2010). Special issue on neuropeptides in stress and addiction: overview. Brain Res 1314: 1–2.
Valentino RJ, Foote SL, Page ME (1993). The locus coeruleus as a site for integrating corticotropin-releasing factor and noradrenergic mediation of stress responses. Ann NY Acad Sci 697: 173–188.
Vranjkovic O, Gasser PJ, Gerndt CH, Baker DA, Mantsch JR (2014). Stress-induced cocaine seeking requires a beta-2 adrenergic receptor-regulated pathway from the ventral bed nucleus of the stria terminalis that regulates CRF actions in the ventral tegmental area. J Neurosci 34: 12504–12514.
Wilensky AE, Schafe GE, Kristensen MP, LeDoux JE (2006). Rethinking the fear circuit: the central nucleus of the amygdala is required for the acquisition, consolidation, and expression of Pavlovian fear conditioning. J Neurosci 26: 12387–12396.
Woulfe JM, Flumerfelt BA, Hrycyshyn AW (1990). Efferent connections of the A1 noradrenergic cell group: a DBH immunohistochemical and PHA-L anterograde tracing study. Exp Neurol 109: 308–322.
Wu Y, Li Y, Yang X, Sui N (2014). Differential effect of beta-adrenergic receptor antagonism in basolateral amygdala on reconsolidation of aversive and appetitive memories associated with morphine in rats. Addict Biol 19: 5–15.
Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, de Lecea L et al (2010). Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc 5: 439–456.
Zhang J, Muller JF, McDonald AJ (2013). Noradrenergic innervation of pyramidal cells in the rat basolateral amygdala. Neuroscience 228: 395–408.
Acknowledgements
We thank The HOPE Center viral vector core (NINDS, P30NS057105), Bakewell Imaging Center, and Karl Deisseroth for the opto-β2AR cDNA. Finally, we also thank the members of the Bruchas laboratory, Robert Gereau IV (WUSTL), Thomas Baranski (WUSTL), Joe Henry Steinbach (WUSTL), and N Gautam (WUSTL) for helpful discussion and technical assistance.
Author contributions
ERS designed and performed experiments, collected and analyzed data, and wrote the manuscript. RA, JGM, and DLB performed experiments and collected data. MRB helped design and oversee experiments and wrote the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
Siuda, E., Al-Hasani, R., McCall, J. et al. Chemogenetic and Optogenetic Activation of Gαs Signaling in the Basolateral Amygdala Induces Acute and Social Anxiety-Like States. Neuropsychopharmacol 41, 2011–2023 (2016). https://doi.org/10.1038/npp.2015.371
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2015.371
This article is cited by
-
Liposaccharide-induced sustained mild inflammation fragments social behavior and alters basolateral amygdala activity
Psychopharmacology (2023)
-
Mechanisms underlying remediation of depression-associated anxiety by chronic N-acetyl cysteine treatment
Psychopharmacology (2020)
-
Noradrenergic circuits in the forebrain control affective responses to novelty
Psychopharmacology (2020)
-
Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects
Nature Communications (2019)
-
Bioresorbable photonic devices for the spectroscopic characterization of physiological status and neural activity
Nature Biomedical Engineering (2019)