Abstract
The amygdala is a major structure that orchestrates defensive reactions to environmental threats and is implicated in hypervigilance and symptoms of heightened arousal in posttraumatic stress disorder (PTSD). The basolateral and centromedial amygdala (CMA) complexes are functionally heterogeneous, with distinct roles in learning and expressing fear behaviors. PTSD differences in amygdala-complex function and functional connectivity with cortical and subcortical structures remain unclear. Recent military veterans with PTSD (n=20) and matched trauma-exposed controls (n=22) underwent a resting-state fMRI scan to measure task-free synchronous blood-oxygen level dependent activity. Whole-brain voxel-wise functional connectivity of basolateral and CMA seeds was compared between groups. The PTSD group had stronger functional connectivity of the basolateral amygdala (BLA) complex with the pregenual anterior cingulate cortex (ACC), dorsomedial prefrontal cortex, and dorsal ACC than the trauma-exposed control group (p<0.05; corrected). The trauma-exposed control group had stronger functional connectivity of the BLA complex with the left inferior frontal gyrus than the PTSD group (p<0.05; corrected). The CMA complex lacked connectivity differences between groups. We found PTSD modulates BLA complex connectivity with prefrontal cortical targets implicated in cognitive control of emotional information, which are central to explanations of core PTSD symptoms. PTSD differences in resting-state connectivity of BLA complex could be biasing processes in target regions that support behaviors central to prevailing laboratory models of PTSD such as associative fear learning. Further research is needed to investigate how differences in functional connectivity of amygdala complexes affect target regions that govern behavior, cognition, and affect in PTSD.
Similar content being viewed by others
INTRODUCTION
The amygdala, a subcortical structure involved in emotion processing and associative fear learning, is central to the pathophysiology of posttraumatic stress disorder (PTSD). Functional connectivity of the amygdala with a variety of other brain structures is disrupted in PTSD at rest (Rabinak et al, 2011; Sripada et al, 2012a) and with task engagement (Fonzo et al, 2010). The role of specific amygdala nuclei and amygdala complexes have been studied intensively in animal models (Phelps et al, 2004). These studies point to specialized roles of basolateral and centromedial amygdala (CMA) complexes in associative fear learning, which is a widely recognized disease model for PTSD (Jovanovic and Ressler, 2010). However, this level of investigation has been largely absent in human sudies, despite the critical role of individual amygdala complexes predicted in PTSD.
Extensive neuroanatomical evidence from rodents (McDonald, 1998), primates (Stefanacci and Amaral, 2002), and humans (Amunts et al, 2005), as well as recent functional neuroimaging evidence in humans (Roy et al, 2009) highlights the amygdala as functionally and structurally heterogeneous. Two broad subdivisions of approximately one dozen nuclei form the basolateral amygdala (BLA) and CMA complexes with differential functional connectivity and separable roles in fear processing (Phelps et al, 2004), known to be dysregulated in PTSD (Armony and Dolan, 2002). The BLA, comprising the lateral, basolateral, basomedial, and basoventral nuclei, affectively evaluates sensory information and is a site of integration with cortical association areas, including those that regulate fear and other emotional responses (Jovanovic and Ressler, 2010). The CMA, comprising the central and medial nuclei, is critical for the orchestration of fear responses via connections with the hypothalamus, basal forebrain, and brainstem (LeDoux, 1998).
Separable roles of the BLA and CMA, previously reported in the animal literature (Pare et al, 1995), were recently clarified in healthy human adults with resting-state fMRI (Roy et al, 2009). The functional connectivity approach uses correlated neural activity between voxels to make inferences about the functional organization of the brain (Biswal et al, 2010). Conveniently, these data can be acquired at rest, unbiased from task demands. The resting-state approach characterizes synchronous patterns of blood-oxygen level dependent (BOLD) activation associated with spontaneous low-frequency fluctuations occurring between voxels or regions (Greicius et al, 2003). In resting-state fMRI analysis of BLA and CMA, cytoarchitechtonically based probability maps of the human amygdala (Amunts et al, 2005) have been used for segmenting the BLA and CMA. The BLA activity was found to be correlated extensively with temporal and frontal cortical regions whereas CMA predicted activity primarily in the striatum (Roy et al, 2009). A similar approach in generalized anxiety disorder (GAD) demonstrated differential connectivity of BLA and CMA (Etkin et al, 2009) and during reward and avoidance learning (Prevost et al, 2011). These initial results in humans demonstrate consistent, replicated delineation of differential connectivity of major amygdala complexes despite their small size and the limited spatial resolution of standard fMRI acquisitions.
In PTSD, resting-state analyses support increased amygdala-insula connectivity and decreased connectivity of the amygdala with the hippocampus and the anterior cingulate cortex (ACC) (Rabinak et al, 2011; Sripada et al, 2012a). However, these investigations were based on whole amygdala seeds; localization of specific alterations to BLA and CMA in PTSD are unknown (Myers and Davis, 2007). Because of the limited empirical knowledge of resting-state functional connectivity in PTSD, we hypothesized altered functional connectivity based on systems for associative fear learning such as contextual fear conditioning and extinction retention (Milad et al, 2007), which are well-recognized models of PTSD (Jovanovic and Ressler, 2010). The prefrontal and sensory-association cortical regions in these systems project primarily to the BLA (Hartley and Phelps, 2010), although recent research has also implicated the CMA in fear learning (LeDoux, 2012). Thus, our first hypothesis was that PTSD would be linked to altered BLA connectivity with key cortical regions such as ventromedial prefrontal cortex (PFC) (Myers and Davis, 2007), ACC (Gilboa et al, 2004), insula (Simmons et al, 2009), and inferior frontal gyrus (IFG) (Morey et al, 2009), and PTSD would be linked to altered CMA connectivity with regions underlying fear expression such as striatum, midbrain, and thalamus (LeDoux, 1998). Our second hypothesis, based on the role of BLA in fear conditioning, was that PTSD would modulate the divergent functional connectivity of BLA and CMA with its target regions. Finally, we proposed that connectivity differences derived from the amygdala as a single structure would produce weaker and less specific connections with target regions than separate BLA and CMA complexes.
PATIENTS AND METHODS
Participants
All participants (n=42) had experienced a DSM-IV Criterion-A trauma and were assigned to a PTSD (n=20) or trauma-exposed control (n=22) group (demographic and clinical information are summarized in Table 1). Participants were recruited between December 2010 and December 2011 from a large registry of US military veterans who served after September 11, 2001 (Dedert et al, 2009). Important exclusion criteria included major neurological disorders, history of brain injury, Axis I psychiatric disorders other than major depression (9 PTSD participants had comorbid MDD), current substance abuse or a history of substance dependence, and contraindications to MRI scanning. All participants provided written informed consent to participate in procedures reviewed and approved by the Institutional Review Boards at Duke University Medical Center and the Durham VA Medical Center. PTSD diagnosis was confirmed using the clinician-administered PTSD Scale on the day of the scan. Of the 47 participants enrolled, three were excluded for having past (lifetime), but not current PTSD, and two were excluded for excessive motion during scanning (>3-mm). Combat and lifetime trauma exposure, psychiatric comorbidities, and medication usage were assessed with structured research instruments (Supplementary Materials).
Data Acquisition
Functional images were acquired on a 3-Tesla GE Signa EXCITE scanner equipped with an 8-channel head coil using spiral-in sampling. The resting-state scan was 378 seconds long (TR:2000 ms, TE:27 ms, flip angle:60, axially oriented slices:34, resolution:3.8-mm3, FOV:256-mm2). The first three volumes were discarded to account for magnetic field stabilization. The spatial resolution of the functional and structural images was similar to published studies examining resting-state connectivity of amygdala complexes (Etkin et al, 2009; Roy et al, 2009; Roy et al, 2013). Participants lay still during the resting-state scan with eyes open while viewing a black fixation cross presented on a gray screen. A high-resolution anatomical T1-weighted image was acquired to aid registration. Resting-state fMRI was collected prior to task-related scanning obtained for a separate study. Signal dropout (susceptibility artifact) in ventral brain areas was assessed by thresholding each voxel’s time-averaged signal to 80% of the mean image intensity to confirm that sufficient signal was present in the amygdala.
Preprocessing
Preprocessing of resting-state data was performed according to established empirically tested procedures (Weissenbacher et al, 2009). Brain extraction, affine registration to the subject’s anatomical image, motion correction, and slice-timing correction were performed using FSL (FMRIB Software Library, FMRIB Centre, University of Oxford, UK). Masks of white matter and cerebrospinal fluid (CSF) created from the anatomical scans were ‘eroded’ by thresholding tissue probability at 80% for CSF and 90% for white matter to minimize the probability of including gray matter (non-white, non-CSF) voxels and applied to each functional scan to extract the average time-series for white matter and CSF. This approach was favored over regressing global mean signal because of the tendency of the latter to introduce spurious anti-correlations (Weissenbacher et al, 2009), distort group differences and correlation patterns (Saad et al, 2012), and remove the dominant signal from the networks of interest (Chen et al, 2012). These time-series and the motion parameters obtained from motion correction were included as regressors. Data were bandpass filtered between 0.008 and 0.1 Hz using 3dBandpass (AFNI, National Institutes of Health, USA). Functional scans were transformed from individual to MNI305 stereotaxic space using MCFLIRT in FSL. Regions of interest for seeds were created using SPM’s Anatomy Toolbox (Eickhoff et al, 2005) with cytoarchitectonically based probability maps of the amygdala instantiated in the Juelich Brain Atlas (Amunts et al, 2005) (Supplementary Materials).
Data Analysis
Subject-level analyses were conducted separately for the left and right hemispheres. The mean time-series for the BLA and CMA were entered as covariates to create connectivity maps for each subject. Thus, unique variance for a given seed, independent of the other complexes, identified voxels with significant functional connectivity. Group-level mixed effect analyses were conducted for each ROI. Multiple comparison correction based on Gaussian Random Field Theory was performed in FSL, which imposes a conservative mean threshold of Z>2.3 for three-dimensional cluster formation and a corrected significance threshold of P<0.05. For voxel clusters showing significant between-group differences in connectivity, average z-scores were extracted from the subject-level connectivity maps to enable follow-up analyses in SPSS. Average z-scores for the ipsilateral amygdala complex were extracted for each cluster as well. Secondary analyses were conducted with data ‘scrubbed’ for motion (Power et al, 2012), with subsets of participants that were either matched on demographic variables or excluded control participants taking serotonergic medications or with a history of MDD (n=2), and statistical control of differences in medication treatment and dosing (Supplementary Materials).
RESULTS
Functional Connectivity in the Trauma-Exposed Control Group
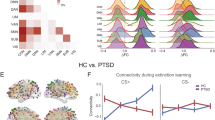
Functional connectivity maps for the trauma-exposed control participants (Figure 1a) were consistent with the results of Roy et al, 2009. Briefly, spontaneous activity in right and left BLA predicted activity in IFG (bilaterally for left BLA and right side only for right BLA), insula, medial temporal lobe, thalamus, bilateral putamen (right BLA only), cerebellum, brainstem, medial and lateral parietal areas, and occipital cortex. Spontaneous activity in the CMA predicted activity in the left insula (left CMA only), right IFG (right CMA only), medial temporal lobe (right side for right CMA, left side for left CMA), bilateral thalamus, bilateral striatum, right superior temporal sulcus (left CMA only), and right cingulate gyrus.
Whole-brain voxel-wise resting-state function connectivity with left and right centromedial amygdala (CMA) seeds (orange overlay) and left and right basolateral amygdala (BLA) seeds (blue overlay) are seen in (a) a trauma-exposed control group and (b) a posttraumatic stress disorder (PTSD) group. Our results in the control group, consistent with results of Roy et al, 2009, showed CMA connectivity to extensive subcortical structures and a few cortical regions, whereas the BLA showed connectivity primarily to cortical structures.
Functional Connectivity in the PTSD Group
In PTSD patients (Figure 1b), spontaneous activity in the left and right BLA predicted activity in multiple cortical and subcortical target regions including the ventromedial PFC (left BLA only), rostral ACC (left BLA only), dorsomedial PFC, left IFG (left BLA only), right striatum (right BLA only), bilateral MTL, right temporal cortex, bilateral insulae, bilateral parietal, and bilateral occipital areas. Spontaneous activity in the left and right CMA seeds predicted activity in the left insula, left IFG (right CMA only), right superior temporal gyrus (left CMA only), inferior temporal gyrus (right CMA only), right cingulate gyrus (left CMA only), posterior cingulate (left CMA only), bilateral MTL, cerebellum, bilateral thalamus, bilateral striatum, and occipital cortex.
Group Differences in Functional Connectivity
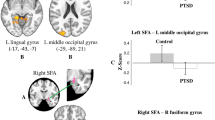
In support of our first hypothesis, the PTSD group showed stronger resting-state functional connectivity than the trauma-exposed control group between the left BLA and a region spanning from pregenual ACC to dorsomedial PFC (Figure 2; Table 2). Likewise, resting-state functional connectivity in the PTSD group was stronger between the right BLA and the dorsal ACC (dACC). The trauma-exposed control group had stronger connectivity than the PTSD group between the right BLA and the left IFG. There were no significant group differences with CMA connectivity.
basolateral amygdala (BLA) connectivity differences between posttraumatic stress disorder (PTSD) and the trauma-exposed control group. (a) The left BLA had stronger connectivity with the pregenual anterior cingulate cortex (ACC) (pgACC) and dorsomedial (dmPFC) in the PTSD group compared with the trauma-exposed control group (orange overlay). (b) The right BLA had stronger connectivity with the dorsal ACC in the PTSD group relative to the trauma-exposed control group (orange overlay). (c) The right BLA had stronger connectivity with the left inferior frontal gyrus (L-IFG) in the trauma-exposed control group than the PTSD group (teal green overlay). All statistical maps represent significance at Z>2.3 (p<0.05; corrected).
Our second hypothesis was tested with the combined group (PTSD+controls) by comparing correlations of BLA and CMA connectivity with the following targets: (1) Left BLA connectivity compared with left CMA connectivity with pregenual ACC/dorsomedial PFC showed an inverse correlation (r=−0.361, p=0.02), (2) BLA versus CMA connectivity to dACC showed a non-significant association (r=−0.198, p=0.210), and (3) BLA versus CMA connectivity to left IFG also revealed an inverse relationship (r=−0.482, p=0.001) (Figure 3). To test whether PTSD modulated this relationship between BLA and CMA functional connectivity, we performed Fisher’s r to z transformation on correlations between BLA and CMA connectivity strengths. The t-tests on correlation strengths did not differ between groups for any targets (p-values >0.05).
Earlier work in rodents and primates established distinct roles for the basolateral amygdala (BLA) and centromedial amygdala (CMA) complexes that we confirmed here in humans. We found a divergent relationship in connectivity strengths (using Fisher’s r to z transformation) of BLA and CMA with select target regions. Three target regions were identified in the main analyses (see Figure 2) as exhibiting differences in BLA connectivity between posttraumatic stress disorder (PTSD) and trauma-exposed control groups. Scatter plots in the combined sample (PTSD+Control) highlight the significant connectivity relationships of BLA (x-axis) and CMA (y-axis) with two of the three target regions: (a) left inferior frontal gyrus (L-IFG) (r=–0.482, p=0.001) shaded in turquoise and (b) the dorsomedial (dm) PFC (r=–.361, p=0.02) shaded in gold. This divergent connectivity relationship between BLA and CMA was not significantly modulated by PTSD (p-values >0.05).
To address our third hypothesis, we investigated the value of partitioning the amygdala into BLA and CMA complexes by repeating the main analyses with a single amygdala seed (spatially combined BLA and CMA). Differential left amygdala connectivity in the PTSD group was observed with a single region in anterior medial PFC. No other group differences in connectivity were identified.
DISCUSSION
In this study, we examined functional connectivity associated with task-free spontaneous neural activity in basolateral and CMA complexes in PTSD and trauma-exposed control groups. Our findings in the trauma-exposed control group supported (see Figure 1) previously published reports of BLA connectivity with cortical regions and CMA connectivity with subcortical regions (Etkin et al, 2009; Roy et al, 2009; Roy et al, 2013). Supporting our first hypothesis, we demonstrated in PTSD that the BLA has stronger connectivity with pregenual ACC/dorsomedial PFC and dACC than trauma-exposed controls (Figure 2a and b). On the other hand, we showed that the trauma-exposed control group has stronger BLA connectivity with the left IFG than the PTSD group (Figure 3). No areas showed altered connectivity to the CMA in PTSD. Second, we confirmed in humans what was previously established in animal studies (Pare et al, 1995). Specifically, the BLA and CMA have divergent connectivity relationships with these three cortical target regions (pregenual ACC/dorsomedial PFC, dACC, left IFG). However, this divergent connectivity relationship was not modulated by PTSD. Finally, we showed that a single amygdala seed provides different and weaker connectivity results than separate BLA and CMA seeds.
Differences in resting-state connectivity of the BLA with various cortical regions in PTSD may be biasing the normal modulation of connectivity between these amygdala complexes and cortical regions during task engagement. Given that BLA and CMA have not been separately investigated in PTSD previously, definite conclusions should be deferred pending confirmatory studies. Nevertheless, several neural systems and processes related to PTSD and engaged by fear conditioning and extinction offer valuable insights. The BLA is strongly implicated in fear generalization and encoding, and retrieval of fear memories, which represent critical behavioral disruptions in PTSD (Jovanovic and Ressler, 2010). Facial expressions of fear, a prototypical innate threat cue in humans, cause hypervigilance in a rare condition of genetically mediated damage to the BLA (Terburg et al, 2012). Indeed, BLA neurons specifically regulate hippocampal neurogenesis triggered by fearful contexts (Kirby et al, 2012). In rats, context-specific freezing supports the role of the BLA in the permanent storage of fear memories (Gale et al, 2004). Furthermore, the BLA is critical to reinstating a fear response to a previously extinguished fear memory (Laurent and Westbrook, 2010), which has been shown to be disrupted in PTSD (Milad et al, 2009).
Failure to learn and recall the extinction memory has been linked to greater amygdala activation in PTSD, but has not been further localized to a specific complex within this functionally heterogeneous structure (Milad et al, 2009). At rest, healthy normal subjects’ BLA activity predicts activity in the hippocampus, parahippocampal gyrus, ventromedial PFC, and insula, whereas spontaneous activity in the CMA predicts activity primarily in striatum, dACC, and insula. Information from multiple sensory modalities that facilitate the encoding of fear memories associated with sensory information (Dolcos et al, 2004) enters the BLA and signals its threat value to cortical targets (Myers and Davis, 2007). Specifically, the ventromedial PFC and IFG incorporate contextual details associated with the threat response (Phelps et al, 2004). The BLA in turn activates the central nucleus, a component of the CMA, which is critical for initiating species-specific defensive reactions via projections to the brainstem, hypothalamus and basal forebrain (Myers and Davis, 2007). Thus, connectivity differences in these major amygdala complexes are consistent with the network of disrupted regions in PTSD. Prevailing models have localized fear learning largely to nuclei in the BLA (Phelps et al, 2004) although recent studies have asserted a possible role for the CMA (LeDoux, 2012). Based on the BLA’s role in fear learning and in integrating information from the cortex, we hypothesized and confirmed selective, disrupted BLA connectivity in PTSD.
LeDoux (LeDoux, 1998) has also shown a strong link of the CMA to fear expression, and more generally to defensive behaviors such as freezing, autonomic arousal, and HPA activation. It is also well established that dACC abnormalities are present in PTSD from a variety of cognitive challenge tasks including extinction recall following fear conditioning (Milad et al, 2009). Our results confirm functional connectivity of CMA and dACC reported by Roy et al (2009) Thus, in addition to postulating CMA connections to the major regions involved in fear expression, it is possible to consider that PTSD might alter the functional connectivity of CMA with dACC. However, primate neuroanatomy shows the cingulate is connected to the basal and lateral nuclei but not to the central and medial nuclei (Freese and Amaral, 2009). Therefore, the functional connections between dACC and CMA must be indirect connections, or CMA connectivity in the human brain departs significantly from the primate and rodent brain. The latter scenario is unlikely, based on phylogeny. While our study found CMA functional connectivity with dACC, this functional connection was not modulated by PTSD.
The cortical targets of the BLA that we found were modulated by PTSD, including pregenual ACC/dorsomedial PFC and dACC, and are strongly implicated in PTSD by behavioral and cognitive challenge tasks (Milad et al, 2009; Morey et al, 2009). These cortical targets have myriad functions, but are also primary components of the default mode and salience networks. The default mode network exhibits reduced coherence during cognitively demanding tasks and is hypothesized to be involved in monitoring of internal (self-related) activity (Greicius et al, 2003), although the amygdala is not a part of the canonical default mode network. Stronger BLA connectivity with critical default mode regions in PTSD, particularly dorsomedial PFC, may reflect an overindulgence of the amygdala with internal monitoring and self-referential thoughts (Mitchell et al, 2005), ostensibly of trauma memories and experiences. This potentiated connectivity of the amygdala with default mode regions that are strongly associated with self-referential thoughts could be mediating elevated anxiety in PTSD during rest, i.e., in the absence of goal-directed cognitive activity. Meanwhile, the BLA shows increased connectivity with the dACC, a component of the salience network. Stronger BLA–dACC connectivity associated with PTSD may be facilitating increased response to stimuli in the environment, even in the absence of salient information. Increased coupling of the BLA with components of both the default mode and salience networks is consistent with findings of increased connectivity between these networks in PTSD (Sripada et al, 2012b).
Our finding that BLA connectivity with left IFG was stronger in the trauma-exposed control group than the PTSD group has multiple interpretations, given the diverse functions of the IFG. In nonclinical populations, IFG promotes effective coping with distracting effects of emotion on cognitive processing (Anticevic et al, 2010), conscious regulation of emotions (Hayes et al, 2010), and response selection/inhibition (Zhang and Li, 2012). Among these functions, IFG activity is typically negatively correlated with amygdala activity (Hayes et al, 2010). However, in PTSD, the IFG shows increased activation when processing trauma-related material (Morey et al, 2009). Persistent anxiety in PTSD may recruit coping mechanisms that engage the IFG to bridle anxiety. However, it is unclear whether engaging the IFG in rest situations is effective in abating anxiety in PTSD. It may be that attention demanding goal-directed activities are an effective strategy for managing anxiety in PTSD. Abrupt increases in trauma-related thoughts and memories frequently coincide with a precipitous decline in goal-directed activity such as job loss or retirement (Schnurr et al, 2005). Future research that directly compares connectivity differences between rest and distraction may offer insights into neural systems involved in holding symptoms of intrusive thoughts and memories at bay through intentional distraction.
The dACC and the ventromedial PFC are active in concert with the amygdala at various stages of fear learning, extinction, and extinction recall (Milad et al, 2009). These two regions have opposite roles in fear conditioning. The dACC is involved in the expression of conditioned fear, whereas the ventromedial PFC shows activation during extinction learning (Myers and Davis, 2007). However, our results in PTSD show both structures have increased connectivity with the BLA, indicating a generalized change in connectivity across this region. This finding is distinct from studies of connectivity differences with the whole amygdala in PTSD, which have found either lower connectivity (Sripada et al, 2012a) or a lack of connectivity differences (Rabinak et al, 2011) between the dACC and amygdala, and no differences in connectivity between ventromedial PFC and amygdala. Together with our findings of negative correlations between BLA and CMA connectivity strengths, the increased connectivity of BLA with dACC and ventromedial PFC supports a unique relationship between BLA and areas critical to fear processing. Increased BLA connectivity with these structures may reflect excessive BLA involvement and a failure of cortical control structures during fear processing in PTSD, but further studies of fear processing in PTSD are needed to elaborate the connectivity relationships of the BLA with the ventromedial PFC and dACC.
The approach of partitioning the amygdala into BLA and CMA produced connectivity patterns that were consistent with the role of the amygdala in associative fear learning, a prevailing disease model for PTSD. The utility of partitioning the amygdala was further substantiated by showing divergent connectivity strengths of BLA and CMA (Figure 3) with the three target regions identified in the main analyses (Figure 2). Although resting-state connectivity of the BLA with cortical targets is altered in PTSD, the relationship between BLA and CMA connectivity to these targets is not further modulated by PTSD, unlike earlier work in GAD (Etkin et al, 2009; Roy et al, 2013). Separate amygdala seeds in GAD showed weakened connectivity of amygdala complexes with their respective targets but stronger connectivity with the targets of other complexes (Etkin et al, 2009; Roy et al, 2013). In contrast, our results in PTSD show alterations are confined to specific clusters of BLA connectivity. Whereas GAD and PTSD are both characterized by anxiety, hypervigilance characterized by increased reactivity to unpredictable threat is unique to PTSD, which may account for the differences in connectivity profiles among these disorders (Grillon et al, 2009). However, this prediction would have to be confirmed by directly comparing patients with PTSD and GAD within a single study.
Limitations
Exclusion of participants taking psychotropic medication has been the accepted orthodoxy, although leaders in the field of PTSD neuroimaging have recently argued for their inclusion (Lanius et al, 2010). Medication use was significantly more common in the PTSD group than the trauma-exposed control group. The use of medications, specifically serotonergic medications, affects connectivity of the amygdala with cortical areas (McCabe and Mishor, 2011). As a result, the use of serotonergic medication by participants in the PTSD group may be biasing results. However, we found no correlations of medication dosage with connectivity strength (Supplementary Material). Two trauma-exposed control participants who had a history of MDD were taking serotonergic medication. Removing these two participants from the analyses did not significantly alter the results (Supplementary Material). In addition, cardiac and respiratory-related fluctuations in BOLD signal were not acquired. Significant correlations have been reported between the cardiac rate and resting BOLD signal time courses, particularly negative correlations in gray matter (Chang et al, 2009). Thus, possible differences in heart rate variability associated with PTSD (Tan et al, 2011) might confound the present results. Our approach conceptualized amygdala complexes as seeds and cortical regions as targets. However, making causal inferences that spontaneous BOLD activity in the amygdala directly or indirectly produced synchronous activity at targets (or vice versa) would be misleading and the directionality of the effects cannot be inferred from the correlational techniques we applied. Moreover, our analyses do not provide information on whether functional connectivity is inhibitory or excitatory. Finally, our results supported some of the structural connections established in animal model investigations. However, significant differences in results were expected and noted between our functional connectivity approach and the postmortem structural mapping in animals.
Conclusions
Our findings show BLA connectivity differences with multiple cortical targets are modulated by PTSD. Each of these cortical targets have been strongly implicated in PTSD, whereas BLA and CMA carry out specialized functions in supporting behaviors central to prevailing laboratory models of PTSD. Thus, differences in resting-state connectivity with BLA and CMA could be biasing how each complex differentially modulates processes in target regions that govern behavior, cognition, and affect in PTSD. Our results may point to potential treatment avenues in PTSD (Felmingham et al, 2007) such as the use of neuro feedback using real-time fMRI to directly alter connectivity strength (Johnston et al, 2010). Fear conditioning and extinction studies should explore differential roles of amygdala complexes in PTSD.
FUNDING AND DISCLOSURE
Funding for Ms Brown, Dr Morey, Ms Haswell and this study was provided by Veterans Health Affairs Merit Grant CX000120 Clinical Services R&D and Veterans Health Affairs Mental Illness Research Education and Clinical Center Grant for Post-Deployment Mental Health. Drs LaBar and McCarthy were funded by the NIH, and Dr Gold was funded by Yale University.
References
Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah N et al (2005). Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol 210: 343–352.
Anticevic A, Repovs G, Barch DM (2010). Resisting emotional interference: brain regions facilitating working memory performance during negative distraction. Cogn Affect Behav Neurosci 10: 159–173.
Armony JL, Dolan RJ (2002). Modulation of spatial attention by fear-conditioned stimuli: an event-related fMRI study. Neuropsychologia 40: 817–826.
Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM et al (2010). Toward discovery science of human brain function. Proc Natl Acad Sci USA 107: 4734–4739.
Chang C, Cunningham JP, Glover GH (2009). Influence of heart rate on the BOLD signal: the cardiac response function. Neuroimage 44: 857–869.
Chen G, Xie C, Ward BD, Li W, Antuono P, Li SJ (2012). A method to determine the necessity for global signal regression in resting-state fMRI studies. Magn Reson Med. 68: 1828–1835.
Dedert EA, Green KT, Calhoun PS, Yoash-Gantz R, Taber KH, Mumford MM et al (2009). Association of trauma exposure with psychiatric morbidity in military veterans who have served since September 11, 2001. J Psychiatr Res 43: 830–836.
Dolcos F, LaBar KS, Cabeza R (2004). Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron 42: 855–863.
Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K et al (2005). A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25: 1325–1335.
Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD (2009). Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch Gen Psychiatry 66: 1361–1372.
Felmingham K, Kemp A, Williams L, Das P, Hughes G, Peduto A et al (2007). Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychol Sci 18: 127–129.
Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB (2010). Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol Psychiatry 68: 433–441.
Freese JL, Amaral DG (2009). Neuroanatomy of the primate amygdala. In: Whalen PJ, Phelps EA (eds.) The Human Amygdala. Guilford Press: New York, NY. pp 3–42.
Gale GD, Anagnostaras SG, Godsil BP, Mitchell S, Nozawa T, Sage JR et al (2004). Role of the basolateral amygdala in the storage of fear memories across the adult lifetime of rats. J Neurosci 24: 3810–3815.
Gilboa A, Shalev AY, Laor L, Lester H, Louzoun Y, Chisin R et al (2004). Functional connectivity of the prefrontal cortex and the amydala in posttraumatic stress disorder. Biol Psychiatry 55: 263–272.
Greicius MD, Krasnow B, Reiss AL, Menon V (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci 100: 253–258.
Grillon C, Pine DS, Lissek S, Rabin S, Bonne O, Vythilingam M (2009). Increased anxiety during anticipation of unpredictable aversive stimuli in posttraumatic stress disorder but not in generalized anxiety disorder. Biol Psychiatry 66: 47–53.
Hartley CA, Phelps EA (2010). Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology 35: 136–146.
Hayes JP, Morey RA, Petty CM, Seth S, Smoski MJ, McCarthy G et al (2010). Staying cool when things get hot: emotion regulation modulates neural mechanisms of memory encoding. Front Hum Neurosci 4: 230.
Johnston S, Boehm S, Healy D, Goebel R, Linden D (2010). Neurofeedback: a promising tool for the self-regulation of emotion networks. Neuroimage 49: 1066–1072.
Jovanovic T, Ressler KJ (2010). How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry 167: 648–662.
Kirby ED, Friedman AR, Covarrubias D, Ying C, Sun WG, Goosens KA et al (2012). Basolateral amygdala regulation of adult hippocampal neurogenesis and fear-related activation of newborn neurons. Mol Psychiatry 17: 527–536.
Lanius RA, Brewin CR, Bremner JD, Daniels JK, Friedman MJ, Liberzon I et al (2010). Does neuroimaging research examining the pathophysiology of posttraumatic stress disorder require medication-free patients? J Psychiatry Neurosci 35: 80–89.
Laurent V, Westbrook RF (2010). Role of the basolateral amygdala in the reinstatement and extinction of fear responses to a previously extinguished conditioned stimulus. Learn Mem 17: 86–96.
LeDoux J (1998). Fear and the brain: where have we been, and where are we going? Biol Psychiatry 44: 1229–1238.
LeDoux J (2012). Rethinking the emotional brain. Neuron 73: 653–676.
McCabe C, Mishor Z (2011). Antidepressant medications reduce subcortical-cortical resting-state functional connectivity in healthy volunteers. Neuroimage 57: 1317–1323.
McDonald AJ (1998). Cortical pathways to the mammalian amygdala. Prog Neurobiol 55: 257–332.
Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB et al (2009). Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 66: 1075–1082.
Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL (2007). Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry 62: 446–454.
Mitchell JP, Banaji MR, Macrae CN (2005). The link between social cognition and self-referential thought in the medial prefrontal cortex. J Cogn Neurosci 17: 1306–1315.
Morey RA, Dolcos F, Petty CM, Cooper DA, Hayes JP, LaBar KS et al (2009). The role of trauma-related distractors on neural systems for working memory and emotion processing in posttraumatic stress disorder. J Psychiatr Res 43: 809–817.
Myers KM, Davis M (2007). Mechanisms of fear extinction. Mol Psychiatry 12: 120–150.
Pare D, Smith Y, Pare JF (1995). Intra-amygdaloid projections of the basolateral and basomedial nuclei in the cat: phaseolus vulgaris-leucoagglutinin anterograde tracing at the light and electron microscopic level. Neuroscience 69: 567–583.
Phelps EA, Delgado MR, Nearing KI, LeDoux JE (2004). Extinction learning in humans: role of the amygdala and vmPFC. Neuron 43: 897–905.
Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59: 2142–2154.
Prevost C, McCabe JA, Jessup RK, Bossaerts P, O’Doherty JP (2011). Differentiable contributions of human amygdalar subregions in the computations underlying reward and avoidance learning. Eur J Neurosci 34: 134–145.
Rabinak CA, Angstadt M, Welsh RC, Kenndy AE, Lyubkin M, Martis B et al (2011). Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Front Psychiatry 2: 62.
Roy AK, Fudge JL, Kelly C, Perry JS, Daniele T, Carlisi C et al (2013). Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry 52: 290–299.
Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K et al (2009). Functional connectivity of the human amygdala using resting state fMRI. Neuroimage 45: 614–626.
Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A et al (2012). Trouble at rest: how correlation patterns and group difference become distorted after global signal regression. Brain Connect 2: 25–32.
Schnurr PP, Lunney CA, Sengupta A, Spiro A 3rd (2005). A longitudinal study of retirement in older male veterans. J Consult Clin Psychol 73: 561–566.
Simmons AN, Strigo IA, Matthews SC, Paulus MP, Stein MB (2009). Initial evidence of a failure to activate right anterior insula during affective set shifting in posttraumatic stress disorder. Psychosom Med 71: 373–377.
Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC et al (2012a). Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci 37: 110069.
Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS et al (2012b). Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom Med 74: 904–911.
Stefanacci L, Amaral DG (2002). Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. J Comp Neurol 451: 301–323.
Tan G, Dao TK, Farmer L, Sutherland RJ, Gevirtz R (2011). Heart rate variability (HRV) and posttraumatic stress disorder (PTSD): a pilot study. Appl Psychophysiol Biofeedback 36: 27–35.
Terburg D, Morgan BE, Montoya ER, Hooge IT, Thornton HB, Hariri AR et al (2012). Hypervigilance for fear after basolateral amygdala damage in humans. Transl Psychiatry 2: e115.
Weissenbacher A, Kasess C, Gerstl F, Lanzenberger R, Moser E, Windischberger C (2009). Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage 47: 1408–1416.
Zhang S, Li CSR (2012). Functional networks for cognitive control in a stop signal task: independent component analysis. Hum Brain Mapp 33: 89–104.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Supplementary information
Rights and permissions
About this article
Cite this article
Brown, V., LaBar, K., Haswell, C. et al. Altered Resting-State Functional Connectivity of Basolateral and Centromedial Amygdala Complexes in Posttraumatic Stress Disorder. Neuropsychopharmacol 39, 351–359 (2014). https://doi.org/10.1038/npp.2013.197
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2013.197
Keywords
This article is cited by
-
Functional connectivity of amygdala subnuclei in PTSD: a narrative review
Molecular Psychiatry (2023)
-
Large-scale functional hyperconnectivity patterns in trauma-related dissociation: an rs-fMRI study of PTSD and its dissociative subtype
Nature Mental Health (2023)
-
Inhibition of phosphodiesterase-4 mitigates stress-re-stress-paradigm induced mitochondrial perturbations in rats exhibiting PTSD-like symptoms
Neuroscience and Behavioral Physiology (2023)
-
Trait anxiety is related to Nx4’s efficacy on stress-induced changes in amygdala-centered resting state functional connectivity: a placebo-controlled cross-over trial in mildly to moderately stressed healthy volunteers
BMC Neuroscience (2022)
-
An exploratory study of resting-state functional connectivity of amygdala subregions in posttraumatic stress disorder following trauma in adulthood
Scientific Reports (2022)