Abstract
Pharmacological inactivation of the granular insular cortex is able to block nicotine-taking and -seeking behaviors in rats. In this study, we explored the potential of modulating activity in the insular region using electrical stimulation. Animals were trained to self-administer nicotine (0.03 mg/kg per infusion) under a fixed ratio-5 (FR-5) schedule of reinforcement followed by a progressive ratio (PR) schedule. Evaluation of the effect of stimulation in the insular region was performed on nicotine self-administration under FR-5 and PR schedules, as well on reinstatement of nicotine-seeking behavior induced by nicotine-associated cues or nicotine-priming injections. The effect of stimulation was also examined in brain slices containing insular neurons. Stimulation significantly attenuated nicotine-taking, under both schedules of reinforcement, as well as nicotine-seeking behavior induced by cues and priming. These effects appear to be specific to nicotine-associated behaviors, as stimulation did not have any effect on food-taking behavior. They appear to be anatomically specific, as stimulation surrounding the insular region had no effect on behavior. Stimulation of brain slices containing the insular region was found to inactivate insular neurons. Our results suggest that deep brain stimulation to modulate insular activity should be further explored.
Similar content being viewed by others
INTRODUCTION
Tobacco-related diseases are a major population health issue resulting in 5 million deaths per year worldwide, with this number expected to grow to 10 million by 2025 (Proctor, 2004). Although several pharmacotherapies are currently available for smoking cessation, there is still a relatively high rate of relapse among individuals motivated to quit (Le Foll and George, 2007; Rigotti, 2002). This relapse rate stresses the need for the discovery of novel treatments targeting both a reduction in smoking behavior and the relapse rate.
The insula has been the object of considerable recent interest both in the general sense of its overall role in the brain (Craig, 2009, 2010) and its specific role in the neurocircuitry of addiction (Koob and Volkow, 2010). Recent findings demonstrated a correlation between stroke-induced insular damage and a disruption of tobacco addiction, characterized by the ease with which immediate smoking cessation was achieved without craving or relapse (Naqvi et al, 2007). Although this effect of insular damage was not observed in another publication (Bienkowski et al, 2010), others have gone as far as to suggest that unintentional abrupt smoking cessation may be a unique lesion localizer (Hefzy et al, 2011). Subsequent work utilizing animal models has shown insular involvement in different aspects of addictive behavior for various addictive substances (Contreras et al, 2007, 2012; Forget et al, 2010a; Hollander et al, 2008; Scott and Hiroi, 2011). However, even before the findings by Naqvi et al (2007), numerous functional imaging studies reported insular activation during subjective drug urges (Naqvi and Bechara, 2009).
Previous work by our group demonstrated that reversibly inactivating the dorsal granular region of the insular cortex in rats, by local infusion of a baclofen/muscimol mixture, decreased nicotine self-administration (SA) under both fixed ratio (FR) and progressive ratio (PR) schedules of reinforcement and also decreased the reinstatement of nicotine-seeking behavior induced by a nicotine-associated cue or a priming injection of nicotine (Forget et al, 2010a). Importantly, this inactivation had no effect on food-taking behavior assessed as a control.
Deep brain stimulation (DBS) is currently being examined for the treatment of various psychiatric disorders (Goodman and Alterman, 2012; Holtzheimer and Mayberg, 2011; Krack et al, 2010; Ward et al, 2010), including addiction (Halpern et al, 2011; Luigjes et al, 2012). Several reports have established the potential of DBS in the region of the nucleus accumbens (NAcc) in human subjects for the treatment of heroin (Zhou et al, 2011), alcohol (Kuhn et al, 2007, 2011; Muller et al, 2009), and nicotine (Kuhn et al, 2009; Mantione et al, 2010). Animal studies have corroborated these findings with DBS of the NAcc shell attenuating alcohol-taking (Henderson et al, 2010; Knapp et al, 2009) and cocaine-seeking (Vassoler et al, 2008), whereas DBS of NAcc core has been shown to attenuate alcohol-taking (Knapp et al, 2009) and morphine-induced conditioned place preference (Liu et al, 2008). Animal studies have also demonstrated an attenuation of cocaine-taking with DBS applied to the subthalamic nucleus (Rouaud et al, 2010), the medial prefrontal cortex (Levy et al, 2007), and the lateral habenula (Friedman et al, 2010), and an attenuation of cocaine-seeking with DBS applied to the lateral hypothalamus (Levy et al, 2007; for a review see, Hamani and Temel (2012b)). To our knowledge, the effects of DBS in an animal model of nicotine abuse have not yet been investigated.
Here, we evaluated the effects of electrical stimulation in the insular region on nicotine SA behavior, under both FR and PR schedules, as well as nicotine-seeking behavior reinstated by nicotine-associated cue presentation or nicotine-priming injection. We also evaluated the effects of electrical stimulation on food SA behavior, as a control. Finally, we examined the effect of stimulation on brain slices containing insular neurons.
MATERIALS AND METHODS
Animals
Naïve male Long–Evans rats initially weighing 250–275 g were used for all nicotine SA work. Rats were individually housed in a temperature-controlled environment on a 12 h reverse light/dark cycle (lights off from 0900 to 2100 hours) and received 20 g of food pellets with unlimited water access. Naïve male Sprague–Dawley rat pups (P23–24) were used for the electrophysiology work. All experimental procedures described were carried out in compliance with the guidelines of the Canadian Council on Animal Care and were reviewed and approved by the local Animal Care Committees.
Initial training procedures and surgical techniques for nicotine SA were similar to those reported previously (Forget et al, 2010a, 2010b; Gamaleddin et al, 2012; Le Foll et al, 2011; Yan et al, 2012). Animals were initially trained on a schedule in which each lever press resulted in the delivery of a 45-mg food pellet (continuous reinforcement, no cues associated with food delivery). Once trained, each animal was surgically prepared with a chronic IV catheter implanted in the jugular vein; the catheter exited between the scapulae. Surgery was performed under anesthesia induced by xylazine (10 mg/kg, intraperitoneally) and ketamine hydrochloride (75 mg/kg, intraperitoneally). Incision sites were infiltrated with the local anesthetic bupivacaine (0.125%). Buprenorphine was given for postoperative analgesia (0.01 mg/kg, subcutaneously), and a single dose of penicillin (30 000 U, intramuscularly) was administered before surgical procedures. Animals were allowed to recover for a 1-week period before drug SA began.
Drugs
(−)Nicotine hydrogen tartrate (Sigma-Aldrich, St Louis, MO) was dissolved in saline, the pH was adjusted to 7.0 (±0.2), and the solution was filtered through a 0.22 mm syringe filter (Fisher Scientific, Pittsburgh, PA) for sterilization purposes. All nicotine doses are reported as free base concentrations. Nicotine was administered intravenously in a volume of 100 μl/kg/injection or subcutaneously in a volume of 1 ml/kg.
Acquisition of the Nicotine or Food SA
SA sessions were carried out in experimental chambers equipped with two levers (Med Associates, St Albans, VT). The start of the session was signaled by illumination of a house light; switching off this light indicated the time-out period, during which time lever responding was recorded but had no consequences. Rapid delivery of the SA drug (1 s delivery time) was achieved with Med Associates Model PHM-104 pumps. Unit doses were 100 μl/kg; volume adjustments were used to accommodate inter-animal or between-session differences in body weight. Responding on one of the levers (active) resulted in drug delivery when schedule requirements were met, whereas responding on the other lever was recorded, but did not produce any change of lights or drug infusion (active levers were counterbalanced). SA sessions occurred 7 days per week.
In this study, rats acquired nicotine SA under an FR schedule of reinforcement, and the unit dose was 30 μg/kg per infusion of nicotine, expressed as base. Session duration was 60 min, and the time-out period (switching off the house light and illumination of a cue light above the active lever) after each infusion was 1 min. During the first 5 days of acquisition, each lever press during the time-in period resulted in the delivery of an infusion (FR-1); the response requirement was then increased to FR-2 for 3 days and finally to FR-5 (ie, animals were required to make five lever presses for each drug infusion) for 7 days.
The apparatus, the stimuli associated with food delivery, and the schedule of the acquisition for the food SA experiments were exactly the same as described above, except that the rats received a food pellet (45 mg precision pellets; Bioserv, Laurel, MD) instead of a nicotine injection.
Electrode Implantation
After the acquisition phase of the nicotine SA, stereotaxic surgeries to implant electrodes were carried out under the same regimen of anesthetics, analgesic, and antibiotic described above. Insulated stainless-steel electrodes (125 μm diameter with 0.5 mm exposed surface; Plastics One, Roanoke, VA) were bilaterally implanted, at a 10° divergent angle from the vertical, with the exposed surface within the histological boundary of the granular insular cortex (surgical coordinates: anteroposterior −0.40 mm, lateral±4.8 mm, and dorsoventral +6 mm; Paxinos and Watson, 1986). Similar electrodes attached to small screws threaded partially into the skull were used as anodes. Rats were allowed 1 week to recover before the reacquisition of nicotine SA under an FR-5 schedule of reinforcement.
Electrical Stimulation
Stimulation was conducted with a portable device (St Jude Medical model 3510, Plano, TX), connected to the animals through extension cables to a multichannel commutator (Plastics One, Roanoke, VA). Animals were stimulated at 50 μA, 90 μs of pulse width, and 130 Hz. These settings are similar to previous publications both from our laboratory (Hamani et al, 2010a, 2012a) and others (see Introduction). The sham condition consisted of the animals being connected to the stimulation equipment but not receiving any stimulation. The testing of stimulation and sham conditions were counterbalanced in all experiments. In all testing, animals were stimulated 5 min before and throughout the duration of the test session (ie, usually 60 min, except for PR sessions, which lasted 4 h).
Testing under FR
At 1 week after electrode implantation, the nicotine or food SA under the FR-5 schedule of reinforcement was re-established for all rats until stabilization. Rats were considered to have acquired stable SA when they pressed the active lever more than two times the number of times they pressed the inactive lever and received a minimum of 10 reinforcements during 1-h session with <20% variation in the number of reinforcements earned per session during two consecutive sessions.
Two groups of rats (n=12 for nicotine SA, and n=9 for food SA) were tested under the FR-5 schedule of reinforcement with stimulation of the insular region or sham in a counterbalanced manner. Animals were required to achieve stable SA criteria (described above) between testing sessions. Animals were also tested in a session where nicotine was substituted with saline (saline substitution condition) as a positive control measure.
Testing under PR
After the testing under an FR-5 schedule of reinforcement, the nicotine group (n=11) was switched to a PR schedule, wherein the response requirement increased with each successive reinforcement. A separate group was trained and tested under a PR schedule for food SA (n=9). The response requirement progression was based on the formula 5e(0.25 × (inj. number+3))−5, with the first two values replaced by 5 and 10 (modified from Roberts (1992)). Thus, the response requirements for successive reinforcements were 5, 10, 17, 24, 32, 42, 56, 73, 95, 124, 161, 208, and so forth. The break point (BP) was defined as the highest ratio completed before the first 30 min period without a response on the active lever. The PR sessions lasted a maximum of 4 h. The animals were allowed 10 days of SA on the PR schedule before testing began. Animals were tested with stimulation of the insular region or sham in a counterbalanced manner, plus a saline substitution condition for the nicotine SA group.
Extinction
After completion of testing on nicotine SA under FR and PR schedules, rats continued on their nicotine SA sessions under an FR schedule for a minimum of five additional sessions to re-establish a stable baseline level of responding for three consecutive sessions. This was followed by an extinction phase that was conducted by withholding nicotine and its associated cues (the house light remained on during the whole session and there was no presentation of the nicotine-associated cues). Responses on the active or inactive lever were recorded but had no consequences. The criterion for extinction was <20 active lever presses per 1-h session over two consecutive sessions.
Cue-Induced Reinstatement of Nicotine-Seeking
Animals were then tested for the effect of stimulation of the insular region or sham stimulation on cue-induced reinstatement in a counterbalanced, within-subject design (n=10). Testing days were separated by at least three extinction sessions with a stable extinction responding (under the criteria for extinction) over two consecutive sessions. Reinstatement tests were conducted under conditions identical to that of SA, except that (1) a single presentation of the visual cue (light above the active lever on and house light off for 60 s) was delivered response-independently immediately at the start of the session, and (2) responses on the active lever (under an FR-5 schedule) resulted in contingent presentation of the cues (light above the active lever on and house light off for 60 s) without nicotine availability (no infusions). Responses on the inactive lever were recorded but were without consequence. The testing sessions lasted 1 h.
Nicotine-Induced Reinstatement of Nicotine-Seeking
After the cue-induced testing, responding was again extinguished according to the same criteria and subsequently animals were also tested for the effect of stimulation of the insular region or sham stimulation on nicotine-induced reinstatement in a counterbalanced, within-subject design (n=8).
Testing days were separated by at least three extinction sessions with a stable extinction responding over two consecutive sessions. Nicotine priming consisted of a 0.15 mg/kg subcutaneous injection of nicotine 10 min before the test session.
Histological Procedures
After completion of behavioral testing, rats were overdosed with pentobarbital (approximately 350 mg/kg, intraperitoneally). Brains were removed, frozen in methylbutane, and coronal serial sections (15 μm thick) were stained with cresyl violet for the determination of electrode placements. Acceptable histology required that the tip of the electrode lie within the insular region (ie, granular and agranular subgregions) on both sides of the brain. It should also be noted that we utilize the term insular region as we cannot be sure of the exact areas influenced by stimulation, not because we are unaware of the location of electrode tips.
Electrophysiology
The procedure for obtaining brain slices and conducting electrophysiology are described elsewhere (Shin et al., 2007; Hamani et al, 2010b). In brief, male Sprague–Dawley rat pups (P23–24) were anesthetized with isoflurane and decapitated. Brains were sliced 300 μm thick in the coronal orientation using a vibratome (VT 1200S, Leica, IL) in chilled dissecting solution containing (in mM): 87 NaCl, 2.5 KCl, 1.25 NaH2PO4, 7 MgCl2, 0.5 CaCl2, 24 NaHCO3, 25 glucose, and 75 sucrose (oxygenated with 95% O2/5% CO2). Information on preparation of slices and visualization is included in Supplementary Materials and Methods.
Insular neurons were stimulated using a 125-μm diameter biconcentric electrode (FHC, Bowdoin, ME) that was positioned ⩽150 μm from the recorded neuron. Stimulation was monophasic and applied for 30–120 s and set to 130 Hz, 60–90 μs in pulse width and at 100–300 μA of current with a Grass S48 stimulator (Grass Instruments, Rockland, MA) that was coupled to a PSIU6 current isolation unit (Grass Instruments).
Data Analysis
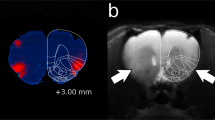
Only rats with correct bilateral placement of the electrodes in the insular region were included for data analysis (n=12; Figure 1), whereas those with incorrect placements were separately analyzed as anatomical controls (n=7; Supplementary Figure S1B). For all testing, relevant data (infusions, pellets, or lever responding) were analyzed using one-way repeated measures (RM) analysis of variance (ANOVA) with Bonferroni comparisons as a post hoc test.
Histological reconstruction of electrode placements in the insula. Black dots indicate locations of electrode tips from the animals that were included in statistical analysis. The number beside each reconstructed image indicates the distance (in mm) from bregma. Schematic figure was published in The Rat Brain in Stereotaxic Coordinates (Paxinos et al, 1986). GI, granular insula; DI, dysgranular insula; AI, agranular insula.
RESULTS
Effect of Stimulation on Nicotine and Food SA under an FR5 schedule
The one-way RM ANOVA performed on the number of infusions obtained during nicotine SA sessions (Figure 2a) showed a significant effect of treatment (F(3,11)=12.35, P<0.0001), and Bonferroni comparisons indicated that stimulation of the insular region during the session or substitution of nicotine with saline both significantly reduced the number of infusions compared with sham stimulation (P<0.05 stim vs sham; P<0.001 saline vs sham). Comparisons also revealed that infusions during sham sessions were not significantly different from baseline (P>0.05, sham vs baseline) and that saline substitution resulted in significantly fewer infusions compared with stimulation (P<0.01, stim vs saline).
Effects of stimulation of the insular region on nicotine (a) or food (b) self-administration under a fixed ratio-5 schedule of reinforcement (n=12 and 9, respectively). Data are expressed as means (±s.e.m.) of the number of injections or food pellet deliveries during regular self-administration (baseline), animals being connected to the stimulator but not stimulated (sham), stimulation of the insular region (stim), or the substitution of nicotine infusions with saline (saline). The sham and stim treatments were counterbalanced in both experiments. *P<0.05; ***P<0.001 vs sham; Bonferroni multiple comparisons following repeated-measures one-way analysis of variance (ANOVA). NS, nonsignificant.
The one-way RM ANOVA performed on the number of pellets obtained during the food SA sessions (Figure 2b) showed no significant effect of treatment (F(2,8)=3.28, P>0.05).
The one-way RM ANOVA performed on the number of infusions obtained during the sessions by animal with misplaced electrodes (Supplementary Figure S1A) showed a significant effect of treatment (F(3,6)=15.78, P<0.0001). However, Bonferroni comparisons indicated that stimulation in the insular region during the session had no significant effect, whereas substitution of nicotine by saline significantly reduced the number of infusions compared with sham (P>0.05 stim vs sham; P<0.001 vs saline vs sham).
Stimulation of the insular region significantly decreased nicotine SA but not food SA, whereas stimulation outside this region had no effect.
Effect of Stimulation on Nicotine and Food SA under a PR schedule
The ANOVA performed on the number of nicotine injections that the rats received before 30 min of inactivity (BP; Figure 3a) showed a main effect of treatment (F(3,10)=14.79, P<0.0001) and group comparisons indicated that stimulation of the insular region or substitution of nicotine by saline significantly reduced the BP compared with sham (P<0.01 stim vs sham; P<0.001 saline vs sham). BPs during sham sessions were not significantly different from baseline (P>0.05 sham vs baseline), but saline substitution resulted in significantly lower BPs compared with stimulation (P<0.05 stim vs saline).
Effects of stimulation of the insular region on nicotine (a) or food (b) self-administration under a progressive ratio schedule of reinforcement (wherein the response requirement increased with each successive injection or food pellet delivery; n=11). Data are expressed as means (±s.e.m.) of the number of injections (break point, left y axis) and of the last ratio completed (in the number of lever presses, right y axis) during regular self-administration (baseline), animals being connected to the stimulator but not stimulated (sham), stimulation of the insular region (stim), or the substitution of nicotine infusions with saline (saline). The sham and stim treatments were counterbalanced in both experiments. **P<0.01; ***P<0.001 vs sham; Bonferroni multiple comparisons following repeated-measures one-way analysis of variance (ANOVA). NS, non-significant.
The one-way RM ANOVA performed on the number of pellets obtained during the food SA sessions (Figure 3b) showed no significant effect of treatment (F(2,8)=3.93, P>0.05).
Effect of Stimulation on Nicotine-Associated Cue-Induced Reinstatement of Nicotine Seeking
The ANOVA performed on the active lever presses indicated a main effect of treatment (F(2,9)=21.54, P<0.001). Post hoc analyses showed that the cue presentation during the sham session induced a significant reinstatement of presses on the active lever (P<0.001 sham vs extinction) and that stimulation in the insular region significantly decreased this cue-induced reinstatement (P<0.05 stim vs sham; Figure 4a).
Effect of stimulation of the insular region on (a) nicotine-associated cue- or (b) nicotine-priming-induced reinstatement of nicotine-seeking tests after extinction (n=8 and 6, respectively). Data are expressed as means (±s.e.m.) of the number of lever presses during extinction conditions (extinction) and during sessions with animals being connected to the stimulator but not stimulated (sham) or stimulation of the insular region (stim). The sham and stim treatments were counterbalanced in both experiments. *P<0.05 vs sham; Bonferroni multiple comparisons following repeated-measures one-way analysis of variance (ANOVA).
Effect of Stimulation on Nicotine-Induced Reinstatement of Nicotine Seeking
The ANOVA performed on the active lever presses indicated a main effect of treatment (F(2,9)=17.03, P<0.001). Post hoc analyses showed that nicotine priming before the sham session induced a significant reinstatement of presses on the active lever (P<0.001 sham vs extinction) and that stimulation of the insular region significantly decreased this nicotine-induced reinstatement (P<0.05 stim vs sham; Figure 4b).
Effect of Stimulation on Inactive Lever Presses
The ANOVAs performed for each experiment showed no significant effect of treatment on inactive lever responding (data not shown), indicating that the effect of stimulation was specific to active lever responding.
Electrophysiology
Neurons in the insular cortex (Figure 5a) had a resting membrane potential of −71.7±1.8 mV (n=8), which is consistent with values described elsewhere (Stone et al, 2011). At rest and before stimulation, the cells do not exhibit spontaneous spiking activity in vitro. When high-frequency stimulation (HFS) was applied to these cells, all of them initially exhibited hyperexcitability and fired bursts of action potentials, but then became quiescent during the stimulation period and returned to pre-HFS resting membrane potentials after stimulation was turned off (post-HFS=−69.7±3.1 mV; n=8; P=0.646). Interestingly, we observed two different inactivating responses of insular neurons to HFS. In three neurons, HFS induced a burst of action potentials, which quickly inactivated concurrently with a rapid repolarization of the resting membrane potential; the inactivation of spike activity occurred within 1 s of HFS even though stimulation remained on (Figure 5b). In contrast, five other recorded neurons became quiescent from HFS by undergoing a depolarization block phenomenon (Figure 5c). These cells depolarized by 29.0±2.9 mV during HFS and maintained this potential throughout the stimulation period. After the stimulation was terminated, insular neurons repolarized to pre-HFS values. All of these cells, however, had similar resting membrane potentials (−72.3±1.0 mV for fast inactivating, −71.3±1.9 mV for depolarization blocked cells, P=0.813), morphology under IR-DIC, regular spiking activity with current injection, and input resistance (166.7±18.7 mΩ for fast inactivating, 174.4±28.7 mΩ for depolarization blocked cells, P=0.910; Figure 5a).
(a) Responses to 500 pA negative and positive current injection to insular neurons show regular spiking activity at depolarized potentials. These cells were identified and recorded using infrared differential interference contrast (IR-DIC0 optics and a representative neuron is shown on the right. The black asterisk represents an insular neuron, whereas the white asterisk represents the position of the stimulating electrode. When high-frequency stimulation (HFS) (130 Hz, 60–90 μs, 30–120 s) was applied to insular neurons, all of these neurons became quiescent. However, the inactivation was induced by fast inactivation in three cells (b) and by a depolarization block in five others (c). Traces represent a sample size of eight insular neurons. The color reproduction of this figure is available on the Neuropsychopharmacology Journal online.
DISCUSSION
The results of this study demonstrate that bilateral electrical stimulation of the insular region significantly decreases nicotine SA under two schedules of reinforcement and attenuates the reinstatement of nicotine-seeking behavior induced by either nicotine-associated cues or nicotine-priming injections. In contrast, there was no effect of stimulation on lever pressing for food SA and no effect of stimulation of the area surrounding the insular region on nicotine SA under an FR-5 schedule of reinforcement.
The decreases observed in both nicotine-taking and -seeking are similar to the effects noticed with the inactivation of the granular insular cortex using a baclofen/muscimol mixture (Forget et al, 2010a). This result suggests that a functional target inactivation may be one potential result of HFS in the insular region. The observed electrophysiological effects provide some correlative evidence in support of this suggestion, as all neurons examined showed inactivation, although two different responses were observed. At present, it is uncertain whether the two responses may to be related to two different populations of neurons. Given that all of these cells had similar resting membrane potentials, morphology under IR-DIC, regular spiking activity with current injection, and input resistance, we speculate that the same type of insular neurons can respond to HFS by becoming inactive in different ways, depending on their ion channel composition. For instance, differences in the sensitivity of delayed inward-rectifying K+ (KDir) channels or Ca2+-dependent BK K+ channels to voltage potentials could underlie the fast inactivating responses. Conversely, the cells that underwent a depolarization block might have become quiescent owing to the inactivation of Na+-voltage-gated channels. These neurons required longer durations of HFS to elicit this response. Further work would be required to examine the respective ion channel compositions of the two neuronal populations observed here.
It must be noted that although decreases in nicotine SA and reinstatement were observed both in our previously published pharmacological inactivation of the granular insular cortex and in the electrical stimulation conducted here, the magnitude of this decrease appears to differ in the two cases. This suggests that the effect of stimulation in the insular region, specifically at the parameters conducted in this study, may not produce the same functional inactivation as baclofen/muscimol infusions. It should be noted that although the electrophysiology data suggest that insular inactivation is a potential result of in vivo stimulation in the region, it cannot be determined whether this is the actual mechanism occurring in our behavioral effects. Other brain areas may also have been influenced by HFS and the behavioral effect observed may be consequent to changes at a distance from the stimulated target and not merely local inhibition (McCracken and Grace, 2007).
The neurocircuitry underlying the insula’s involvement in addiction has only recently begun to be explored. The granular insular cortex’s primary function is to map affective bodily feelings, specifically those critical to homeostasis and survival (Craig, 2009), which include the bodily sensations produced by nicotine. The insula has interconnections with the amydala (Allen et al, 1991), the orbitofrontal cortex (OFC), and the ventromedial prefrontal cortex (vmPFC; Hurley et al, 1991), which also receive dopaminergic input from the ventral–tegmental area that can be released by nicotine’s central effects. The theory proposed by Naqvi and Bechara (2010) is that both the central and peripheral effects of nicotine are involved in the conscious pleasure produced by smoking. Smoking-related cues are posited to result in interconnections from the amygdala and OFC/vmPFC triggering a representation in the insula of the bodily sensations produced by nicotine and subsequently resulting in insular projections to the NAcc motivating drug-seeking behavior (Naqvi and Bechara, 2010). It is through these two pathways that the insula is posited to be involved in both nicotine-taking and -seeking behaviors. However, it should be noted that recent evidence suggests the lateral habenula may also be involved in conveying information from the anterior insular cortex to midbrain monoaminergic centers (Kim and Lee, 2012).
Limited work has been carried out to identify specific receptor populations or molecular changes in the insula associated with nicotine SA. Antagonism of hypocretin-1 receptors present on insular neurons has been demonstrated to decrease nicotine-taking (Hollander et al, 2008) in a rodent SA model. In addition, incubation of nicotine seeking has been associated with enhanced protein kinase A-regulated signaling of dopamine- and cAMP-regulated phosphoprotein of 32 kDa in the insula, suggesting that amplified dopaminergic signaling in this area is critical to the incubation (Abdolahi et al, 2010). This is of significant interest as the insula has been noted as an extra-striatal site of unusually high dopamine transmission (Jones et al, 1986). Further work should be conducted to better understand the various receptor populations and molecular changes involved in the insula’s role in nicotine SA.
Regardless of the underlying neurocircuitry involved, the fact that electrical stimulation in the insular region attenuates behaviors relevant to nicotine abuse is of considerable interest both from a basic and from a clinical perspective. DBS is a technique that is widely used in Parkinson’s disease (Bronstein et al, 2011) and a few groups have reported positive effects of DBS of the NAcc for smoking cessation (Kuhn et al, 2009; Mantione et al, 2010). Other potential methods for electrically modulating insular activity, such as repetitive transcranial magnetic stimulation, should also be considered.
The role of the insula in addiction has only recently begun to be explored, yet the findings thus far point towards it being a crucial structure for different addictive behaviors across various addictive substances. More work is required to uncover other potential treatments, pharmacological or otherwise, which may target this critical brain area.
References
Abdolahi A, Acosta G, Breslin FJ, Hemby SE, Lynch WJ (2010). Incubation of nicotine seeking is associated with enhanced protein kinase A-regulated signaling of dopamine- and cAMP-regulated phosphoprotein of 32 kDa in the insular cortex. Eur J Neurosci 31: 733–741.
Allen GV, Saper CB, Hurley KM, Cechetto DF (1991). Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol 311: 1–16.
Bienkowski P, Zatorski P, Baranowska A, Ryglewicz D, Sienkiewicz-Jarosz H (2010). Insular lesions and smoking cessation after first-ever ischemic stroke: a 3-month follow-up. Neurosci Lett 478: 161–164.
Bronstein JM, Tagliati M, Alterman RL, Lozano AM, Volkmann J, Stefani A et al (2011). Deep brain stimulation for Parkinson disease: an expert consensus and review of key issues. Arch Neurol 68: 165.
Contreras M, Billeke P, Vicencio S, Madrid C, Perdomo G, Gonzalez M et al (2012). A role for the insular cortex in long-term memory for context-evoked drug craving in rats. Neuropsychopharmacology 37: 2101–2108.
Contreras M, Ceric F, Torrealba F (2007). Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science 318: 655–658.
Craig AD (2009). How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci 10: 59–70.
Craig AD (2010). Once an island, now the focus of attention. Brain Struct Funct 214: 395–396.
Forget B, Pushparaj A, Le Foll B (2010a). Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biol Psychiatry 68: 265–271.
Forget B, Wertheim C, Mascia P, Pushparaj A, Goldberg SR, Le Foll B (2010b). Noradrenergic alpha1 receptors as a novel target for the treatment of nicotine addiction. Neuropsychopharmacology 35: 1751–1760.
Friedman A, Lax E, Dikshtein Y, Abraham L, Flaumenhaft Y, Sudai E et al (2010). Electrical stimulation of the lateral habenula produces enduring inhibitory effect on cocaine seeking behavior. Neuropharmacology 59: 452–459.
Gamaleddin I, Wertheim C, Zhu AZ, Coen KM, Vemuri K, Makryannis A et al (2012). Cannabinoid receptor stimulation increases motivation for nicotine and nicotine seeking. Addict Biol 17: 47–61.
Goodman WK, Alterman RL (2012). Deep brain stimulation for intractable psychiatric disorders. Annu Rev Med 63: 511–524.
Halpern CH, Torres N, Hurtig HI, Wolf JA, Stephen J, Oh MY et al (2011). Expanding applications of deep brain stimulation: a potential therapeutic role in obesity and addiction management. Acta Neurochir (Wien) 153: 2293–2306.
Hamani C, Diwan M, Isabella S, Lozano AM, Nobrega JN (2010a). Effects of different stimulation parameters on the antidepressant-like response of medial prefrontal cortex deep brain stimulation in rats. J Psychiatr Res 44: 683–687.
Hamani C, Dubiela FP, Soares JC, Shin D, Bittencourt S, Covolan L et al (2010b). Anterior thalamus deep brain stimulation at high current impairs memory in rats. Exp Neurol 225: 154–162.
Hamani C, Machado DC, Hipolide DC, Dubiela FP, Suchecki D, Macedo CE et al (2012a). Deep brain stimulation reverses anhedonic-like behavior in a chronic model of depression: role of serotonin and brain derived neurotrophic factor. Biol Psychiatry 71: 30–35.
Hamani C, Temel Y (2012b). Deep brain stimulation for psychiatric disease: contributions and validity of animal models. Sci Transl Med 4: 142–148.
Hefzy H, Silver RW, Silver B (2011). The no smoking sign—insular infarction. J Neuroimag 21: e169–e170.
Henderson MB, Green AI, Bradford PS, Chau DT, Roberts DW, Leiter JC (2010). Deep brain stimulation of the nucleus accumbens reduces alcohol intake in alcohol-preferring rats. Neurosurg Focus 29: E12.
Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ (2008). Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci USA 105: 19480–19485.
Holtzheimer PE, Mayberg HS (2011). Deep brain stimulation for psychiatric disorders. Annu Rev Neurosci 34: 289–307.
Hurley KM, Herbert H, Moga MM, Saper CB (1991). Efferent projections of the infralimbic cortex of the rat. J Comp Neurol 308: 249–276.
Jones MW, Kilpatrick IC, Phillipson OT (1986). The agranular insular cortex: a site of unusually high dopamine utilisation. Neurosci Lett 72: 330–334.
Kim U, Lee T (2012). Topography of descending projections from anterior insular and medial prefrontal regions to the lateral habenula of the epithalamus in the rat. Eur J Neurosci 35: 1253–1269.
Knapp CM, Tozier L, Pak A, Ciraulo DA, Kornetsky C (2009). Deep brain stimulation of the nucleus accumbens reduces ethanol consumption in rats. Pharmacol Biochem Behav 92: 474–479.
Koob GF, Volkow ND (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35: 217–238.
Krack P, Hariz MI, Baunez C, Guridi J, Obeso JA (2010). Deep brain stimulation: from neurology to psychiatry? Trends Neurosci 33: 474–484.
Kuhn J, Bauer R, Pohl S, Lenartz D, Huff W, Kim EH et al (2009). Observations on unaided smoking cessation after deep brain stimulation of the nucleus accumbens. Eur Addict Res 15: 196–201.
Kuhn J, Grundler TO, Bauer R, Huff W, Fischer AG, Lenartz D et al (2011). Successful deep brain stimulation of the nucleus accumbens in severe alcohol dependence is associated with changed performance monitoring. Addict Biol 16: 620–623.
Kuhn J, Lenartz D, Huff W, Lee S, Koulousakis A, Klosterkoetter J et al (2007). Remission of alcohol dependency following deep brain stimulation of the nucleus accumbens: valuable therapeutic implications? J Neurol Neurosurg Psychiatry 78: 1152–1153.
Le Foll B, Chakraborty-Chatterjee M, Lev-Ran S, Barnes C, Pushparaj A, Gamaleddin I et al (2011). Varenicline decreases nicotine self-administration and cue-induced reinstatement of nicotine-seeking behaviour in rats when a long pretreatment time is used. Int J Neuropsychopharmacol 15: 1265–1274.
Le Foll B, George TP (2007). Treatment of tobacco dependence: integrating recent progress into practice. Can Med Assoc J 177: 1373–1380.
Levy D, Shabat-Simon M, Shalev U, Barnea-Ygael N, Cooper A, Zangen A (2007). Repeated electrical stimulation of reward-related brain regions affects cocaine but not ‘natural’ reinforcement. J Neurosci 27: 14179–14189.
Liu HY, Jin J, Tang JS, Sun WX, Jia H, Yang XP et al (2008). Chronic deep brain stimulation in the rat nucleus accumbens and its effect on morphine reinforcement. Addict Biol 13: 40–46.
Luigjes J, van den Brink W, Feenstra M, van den Munckhof P, Schuurman PR, Schippers R et al (2012). Deep brain stimulation in addiction: a review of potential brain targets. Mol Psychiatry 17: 572–583.
Mantione M, van de Brink W, Schuurman PR, Denys D (2010). Smoking cessation and weight loss after chronic deep brain stimulation of the nucleus accumbens: therapeutic and research implications: case report. Neurosurgery 66: E218 discussion E218.
McCracken CB, Grace AA (2007). High-frequency deep brain stimulation of the nucleus accumbens region suppresses neuronal activity and selectively modulates afferent drive in rat orbitofrontal cortex in vivo. J Neurosci 27: 12601–12610.
Muller UJ, Sturm V, Voges J, Heinze HJ, Galazky I, Heldmann M et al (2009). Successful treatment of chronic resistant alcoholism by deep brain stimulation of nucleus accumbens: first experience with three cases. Pharmacopsychiatry 42: 288–291.
Naqvi NH, Bechara A (2009). The hidden island of addiction: the insula. Trends Neurosci 32: 56–67.
Naqvi NH, Bechara A (2010). The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct 214: 435–450.
Naqvi NH, Rudrauf D, Damasio H, Bechara A (2007). Damage to the insula disrupts addiction to cigarette smoking. Science 315: 531–534.
Paxinos G, Watson C (1986) The Rat Brain in Stereotaxic Coordinates 2nd edn. Academic Press: New York.
Proctor RN (2004). The global smoking epidemic: a history and status report. Clin Lung Cancer 5: 371–376.
Rigotti NA (2002). Clinical practice. Treatment of tobacco use and dependence. N Engl J Med 346: 506–512.
Roberts DS (1992). Self-administration of stimulants and serotonergic systems. NIDA Res Monogr 119: 136–140.
Rouaud T, Lardeux S, Panayotis N, Paleressompoulle D, Cador M, Baunez C (2010). Reducing the desire for cocaine with subthalamic nucleus deep brain stimulation. Proc Natl Acad Sci USA 107: 1196–1200.
Scott D, Hiroi N (2011). Deconstructing craving: dissociable cortical control of cue reactivity in nicotine addiction. Biol Psychiatry 69: 1052–1059.
Shin DS, Samoilova M, Cotic M, Zhang L, Brotchie JM, Carlen PL (2007). High frequency stimulation or elevated K+ depresses neuronal activity in the rat entopeduncular nucleus. Neuroscience 149: 68–86.
Stone ME, Maffei A, Fontanini A (2011). Amygdala stimulation evokes time-varying synaptic responses in the gustatory cortex of anesthetized rats. Front Integr Neurosci 5: 3.
Vassoler FM, Schmidt HD, Gerard ME, Famous KR, Ciraulo DA, Kornetsky C et al (2008). Deep brain stimulation of the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking in rats. J Neurosci 28: 8735–8739.
Ward HE, Hwynn N, Okun MS (2010). Update on deep brain stimulation for neuropsychiatric disorders. Neurobiol Dis 38: 346–353.
Yan Y, Pushparaj A, Le Strat Y, Gamaleddin I, Barnes C, Justinova Z et al (2012). Blockade of dopamine d4 receptors attenuates reinstatement of extinguished nicotine-seeking behavior in rats. Neuropsychopharmacology 37: 685–696.
Zhou H, Xu J, Jiang J (2011). Deep brain stimulation of nucleus accumbens on heroin-seeking behaviors: a case report. Biol Psychiatry 69: e41–e42.
Acknowledgements
We acknowledge Mustansir Diwan, Yaroslaw Pryslawsky, Eliane Balbino, Maram Khaled, Munmun Chatterjee, Islam Gamaleddin, and Ed Yee for technical support and training.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
This study was supported in part by a 2009 NARSAD Independent Investigator Award awarded to Dr Le Foll. Dr Hamani is a consultant and has received honoraria from St Jude Medical. Dr Le Foll has received grant and salary support from Pfizer and is a consultant for Richter Pharmaceuticals. AP, WY, DS, BK, and JN have no conflicts of interest to declare.
Additional information
Supplementary Information accompanies the paper on the Neuropsychopharmacology website
Rights and permissions
About this article
Cite this article
Pushparaj, A., Hamani, C., Yu, W. et al. Electrical Stimulation of the Insular Region Attenuates Nicotine-Taking and Nicotine-Seeking Behaviors. Neuropsychopharmacol 38, 690–698 (2013). https://doi.org/10.1038/npp.2012.235
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/npp.2012.235
Keywords
This article is cited by
-
Deep Brain Stimulation for Addictive Disorders—Where Are We Now?
Neurotherapeutics (2022)
-
Functional connectivity of the anterior insula during withdrawal from cigarette smoking
Neuropsychopharmacology (2021)
-
Differential regulation of medium spiny and cholinergic neurons in the nucleus accumbens core by the insular and medial prefrontal cortices in the rat
Pflügers Archiv - European Journal of Physiology (2021)
-
Deep TMS of the insula using the H-coil modulates dopamine release: a crossover [11C] PHNO-PET pilot trial in healthy humans
Brain Imaging and Behavior (2018)
-
Role of the agranular insular cortex in contextual control over cocaine-seeking behavior in rats
Psychopharmacology (2017)