Abstract
Most previous magnetic resonance imaging (MRI) studies of patients with bipolar disorder (BD) report similar hippocampus (HC) volumes across patients and controls, but because patients studied were heterogeneous with respect to course of illness variables and medication status, the conclusions of these studies remain equivocal. Lithium (Li) is the reference-standard drug for BD and its role as an important agent in neuroprotection and neurogenesis has been documented in human and in animal studies. We compared the volume of the HC, hippocampal head (Hh), and body/tail (Hbt) in three groups with no history of medication use before entry into this study: (a) a group of patients treated with Li for 1–8 weeks and then scanned; (b) a group comprised of patients who were unmedicated at the time of scan; and (c) a group of patients treated with either valproic acid or lamotrigine. Healthy age- and sex-matched comparison subjects were also scanned. HC volumes did not differ between the unmedicated and healthy comparison groups. There was a bilateral increase in volumes of HC and Hh in the Li-treated group compared to the unmedicated group, an effect that was apparent even over a brief treatment period. Our study provides further confirmation that Li can exert structural effects on the HC, which are detectable in vivo. The study emphasizes the need to control for even brief exposure to medication in volumetric studies of the HC.
Similar content being viewed by others
INTRODUCTION
The hippocampus (HC) is a key component of fronto-temporal neural networks involved in memory (McKinnon et al, 2007) and in emotional regulation (Mayberg, 1997). As such, it is implicated in the cognitive and affective abnormalities observed in mood disorders. There have been two recent meta-analyses reporting bilateral HC volume reduction in patients with major depressive disorder (MDD) compared to healthy controls using magnetic resonance imaging (MRI; Campbell et al, 2004; Videbech and Ravnkilde, 2004). In contrast, results from studies of persons with bipolar disorder (BD) are heterogeneous. There is, however, a tendency to conclude that HC volume is preserved in patients with BD (eg Geuze et al, 2005), despite research reporting both smaller (Blumberg et al, 2003) and larger (Beyer et al, 2004) HC volumes. As the patients included in these studies were heterogeneous with respect to course of illness variables and medication status, the conclusions of these studies remain equivocal (see Table 1). Medication history, in particular, is an important confounding factor in studies of patients with BD (Dickstein et al, 2005; Strasser et al, 2005; Frazier et al, 2005; Rajkowska, 2002).
Lithium (Li) is the reference-standard drug for acute and prophylactic treatment of BD, although the exact mechanism of its effect is unknown (Bachmann et al, 2005). Nonetheless, its role as an important agent in neuroprotection and neurogenesis has been documented in human and in animal studies. For example, Chen et al (2000) demonstrated enhancement of hippocampus neurogenesis in the dentate gyri of Li-treated mice. In a more recent study, Frey et al (2006) reported that Li increased hippocampus brain-derived neurotrophic factor (BDNF) levels in an animal model of mania. The HC has been implicated as a site for cellular plasticity and Li appears to be involved in this process (Chen and Manji, 2006).
Sassi et al (2002; mean of 27 weeks duration) and Moore et al (2000a; mean of 4 weeks duration) reported increased total gray matter volume following Li exposure. No change in HC volume was apparent in patients treated with Li when compared to patients treated with medication other than Li (Chen et al, 2004) or drug-free patients (Brambilla et al, 2003). Similarly, Sax et al (1999) found no differences in HC volumes between medicated and unmedicated patients with BD. A recent study by Velakoulis et al (2006) found no change in HC volumes of patients experiencing their first-episode of mood-related psychosis; excluding those patients using Li from the data did not change this pattern. In contrast, Beyer et al (2004) found a correlation between Li use and an increase in HC sizes in an older patient population.
Despite the fact that a medication naïve population is critical for understanding the structural changes that may reflect the pathophysiology of BD, the association between HC volumes and treatment has not been previously investigated using controlled exposure to medication in people with BD. We, therefore, used a sample with no history of medication use before entry into the study and compared the HC volumes of patients with BD treated with Li (Li+ group) to those of patients who were either unmedicated (UM group) or treated with one of the anticonvulsants, valproic acid or lamotrigine (AC group).
MATERIALS AND METHODS
Subjects
Twenty-eight subjects (13 men and 15 women) were recruited from the mood disorders clinic at St. Joseph's Healthcare Hamilton. Subjects provided written informed consent in accordance with the Declaration of Helsinki. The study was approved by the Research Ethics Boards of St. Joseph's Hospital (ON, Canada) and Hamilton Health Sciences Corporation (ON, Canada). The diagnosis of BD was confirmed by the Structured Clinical Interview for DSM-IV (SCID; First et al 2001). All patients were medication naïve, having never received psychopharmacological treatment for psychiatric illness before entry into the study. Three groups were examined: (i) a Li+ group comprised of 12 patients (mean age=25.73, SD=6.2; five women) treated with Li for 1 to 8 weeks duration (mean=26.67 days; SD=13.50); (ii) a non-Li group (AC) comprised of seven patients (mean age=25.55, SD=8.5; four women) treated for 1–8 weeks duration (mean=30.29 days; SD=13.61) with valproic acid or lamotrigine and (iii) an unmedicated (UM) group of nine patients (mean age=24.36, SD=8.4; six women) who were either untreated with medication (n=5) or who received medication (ie Celexa, Zoloft, Lamictal, or Li) for less than 5 days (mean=1.33; SD=1.89) at the time of scanning.
The control group comprised of 30 healthy comparison subjects matched to the patients in terms of age (mean age=25.28, SD=7.8) and gender distributions (14 males and 16 females).
Exclusion criteria for patients and comparison subjects were: (i) substance-use related disorder within the past 6 months as determined by the SCID; (ii) lifetime history of substance dependence as measured by the SCID; (iii) posttraumatic stress disorder as determined by the SCID; (iv) use of alcohol or illicit psychoactive substance within 48 h of testing; (v) untreated medical illness such as uncontrolled diabetes or other endocrine disorders; (vi) history of head injury with loss of consciousness; (vii) history of neurological disease; and (viii) past treatment with psychotropic medication, electroconvulsive therapy, transcranial magnetic stimulation or a formal course of psychotherapy. See Table 2 for a summary of demographic and clinical variables.
MRI Image Acquisition and Analysis
Fifty-three of the MRI scans were obtained on a 1.5-T. Sigma GE Genesis-based Echo-Speed scanner (General Electric Medical Systems, Milwaukee, WI) running version 5.7 software and using a standard 30cm circularly polarized head coil. Sagittal anatomic images were acquired by using a 3D/FSPGR/20 sequence (flip angle, 20°; echo delay time in-phase (TE), minimum repetition time (TR), 300 msec; inversion recovery, 300 msec; matrix, 512 × 256; field of view (FOV), 24 cm; scan thickness, 1.2 mm). The remaining five subjects were scanned on a 3-T MRI Sigma GE Genesis (General Electric Medical Systems, Milwaukee, WI). Here, sagittal T-1 weighted images were acquired using a 3D FSPGR-IR sequence, (TR/TE =10.3/2.1 ms, flip angle, 20°, inversion time, 300 ms, and FOV=24 cm, slice thickness=1.2 mm. Briellmann et al (2001) reported no difference in HC volumes measurements in the MRI scans of eight healthy adults performed at 1.5 and 3 T. Hence, we treated the images acquired in our study at 1.5 and 3 T as a single data set.
The AFNI software package (National Institute of Mental Health, Bethesda, Maryland, USA; http://afni.nimh.nih.gov/afni/) was used to analyze these data. Our images were T1-weighted, but we were able to utilize the swap feature in AFNI to create a negative image of the original scan, approximating a T-2 weighted image (eg the alveus which is white on T1-weighted images but looks black using this function). This allowed us to examine the images on both the sagittal, coronal, and axial plane, using the T1-weighted and negative images.
Total Cerebral Volume Measurement
Total cerebral volume (TCV) was defined as the gray and white matter of both hemispheres spanning the midbrain superior to the pons, a border chosen for its easy identification. Here, the inter-rater reliability (intraclass correlation coefficient (ICC)) between two raters was 0.99.
Hippocampal Measurement
A detailed description of the measurement protocol can be found at the website http://physics.stjosham.on.ca/~kaan/HippoProtocol.pdf. The HC was defined anatomically as the hippocampus proper (Ammon's horn), dentate gyrus, and most of the subiculum. The alveus, fimbria, and fornix were excluded from these measurements. The sagittal plane was the primary reference plane where the majority of the traces were made. The coronal and axial planes were used as required. The HC was further subdivided into the HC head (Hh), HC body, and tail (Hbt) (Kim et al, 1994). HC volumes were measured by one rater (KY) with reliability confirmed by a second investigator (VHT), falling within 5% between raters. The ICC values were 0.97 for right HC and 0.99 for left HC. HC volumes were obtained with raters who were blind to group membership.
Statistical Analyses
HC volume data were analyzed using one-way ANOVAs where group (Li+, AC, UM, and Control) was treated as a between-subjects variable. Tukey's honestly significant difference post-hoc test with α set at 0.05 was used for follow-up pairwise comparisons. Volumetric differences across male and female subjects were compared using independent-samples t-test treating sex as a between-subjects variable.
RESULTS
Subject Demographics and Clinical Characteristics
There were no differences in age or sex across the patient and healthy comparison groups (p>0.05). Furthermore, the patient groups did not differ significantly from one another at the time of enrollment with respect to number of previous affective episodes, age at onset of first psychiatric episode, psychosis or duration of psychiatric illness (p>0.05). Baseline scores on the 17-item Hamilton Rating Scale for Depression (HAM-D) and Young Mania Scale (YMS) did not differ significantly across the three patient groups (p<0.05). In subjects for whom handedness data were available (45 of 58 subjects), 96% of controls and 83% of patients (across all three groups) were right handed or ambidextrous. Hence, there were too few left-handed subjects in each group to examine reliably differences in HC volume across left- and right-handed subjects.
Total Cerebral Volume
There was a marginally significant main effect of group on TCV (F(3,57)=2.66, p=0.06). Numerically, healthy comparison subjects had the largest TCVs. When TCV was included in the analyses of HC volume described below, there was no impact on the pattern of results for HC volumes.
Volumetric Differences Between Groups
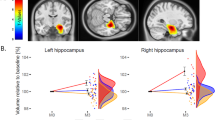
There was a significant main effect of group on left (F(3,57)=3.25, p=0.03) and on right (F(3,57)=3.65, p=0.02) HC volumes (see Table 3). These effects were evident in the Hh (hippocampal head), but not Hbt (hippocampal body-tail). Specifically, there was a main effect of group on left Hh volume (F(3,57)=4.12, p=0.01); this effect was marginally significant for right Hh volume (F(3,57)=2.59, p=0.06). By contrast, no significant group differences emerged for left and right Hbt (p>0.05). Post-hoc testing indicated that the volumes of left (p=0.02) and of right HC (p=0.01), and of left Hh (p<0.01) and right Hh (p=0.04), were larger in the Li+ group than in the UM group. Means and SD for each of these variables are included in Table 3.
Because there was a marginally significant main effect of group on TCV, we repeated our analysis using TCV as a co-variate in an ANCOVA design. All effects remained significant at the p<0.05 level in this analysis, and controlling for TCV enhanced the difference between groups such that the difference in right Hh became significant (p=0.03).
Volumetric Differences Between Men and Women
Healthy men (n=14) had larger right and left HC (p<0.01), right (p<0.01) and left Hh (p=0.04), and left Hbt (p=0.002) than healthy women (n=16). Right Hbt volume, however, did not differ between men and women in the comparison group (p=0.11). After co-varying for TCV, the left Hh and left Hbt volumes were not different between healthy men and women (p=0.08). In the BD group as a whole (n=28), men with BD (n=13) had larger right (p<0.01) and left (p=0.04) Hh volumes than did women with BD (n=15).
Asymmetries in Left and Right Hippocampal Volumes
Right HC and Hh volumes were larger than those of the left in patients (p=0.04) and controls (p<0.01). No such differences, however, emerged for the Hbt in either the patient or comparison groups (p>0.05).
DISCUSSION
The key findings of this study were that medication naïve patients with BD had HC volumes that did not differ from age- and sex-matched healthy comparison subjects, while patients treated with Li had larger total HC and Hh volumes. Co-varying for TCV did not change these results. To our knowledge, this is the first study to examine systematically a medication naïve population with BD compared with short-term treatment with Li and other standard treatments for BD (valproate and lamotrigine). Because of the small sample of patients receiving Li and the relatively short-term duration of treatment, we were unable to examine relations between treatment duration and HC volumes. In the absence of a sufficient preclinical literature examining the time course whereby Li may exert effects on the HC, we are unable to predict whether increases in HC volume following Li would follow a linear function; it seems likely, however, that if the relation between treatment and HC volume is linear over the short-term, the long-term impact of Li treatment would follow a different function.
Most studies comparing patients with BD to healthy comparison subjects report no differences in HC volumes (Hauser et al, 2000; Altshuler et al, 2000; Dickstein et al, 2005; Chen et al, 2004; McDonald et al, 2006; Velakoulis et al, 2006; Brambilla et al, 2003). This contrasts with other neuropsychiatric disorders such as schizophrenia, Alzheimer's disease, post-traumatic stress disorder, borderline personality disorder, and major depressive disorder that are associated with small HC volumes (Campbell and MacQueen, 2006; Geuze et al, 2005) in those with established illness. Following diagnosis, people with BD spend one-third to half their lives with depressive symptoms (Judd et al, 2002, 2003). Patients with BD often have a psychotic element to their illness, and co-morbidity with anxiety disorders, including post-traumatic stress disorder, is common. These features would lead to the prediction of small HC volumes in BD and yet the results of studies to date do not support this (see Table 1). This study may provide one clue to that puzzle, with the finding that Li was associated with larger HC volumes. As studies to date have not controlled for current or previous treatment status, the effect of illness independent of medication is unknown, and it appears that Li may promote volumetric increases in the HC, possibly mitigating against or preventing illness-related changes. In human subjects, an increase in NAA levels after Li treatment in patients with BD (Moore et al, 2000b) suggests that Li may promote neurogenesis and neuroprotection in the HC.
An extensive number of pre-clinical studies have reported neuroprotective effects of Li. Long-term treatment with Li protects primary cultures of rat brain neurons from glutamate-induced, NMDA receptor-mediated excitotoxicity, likely by inhibition of NMDA-receptor-mediated calcium influx, upregulation of anti-apoptotic Bcl-2, downregulation of pro-apoptotic p53 and Bax, and activation of cell (see Shaltiel et al, 2007; Chen and Manji, 2006; Chuang, 2004, 2005 for detailed reviews). Li also induces the expression of BDNF in rat HC (Hashimoto et al, 2002; Frey et al, 2006) and induces activation of its receptor trkB (Rantamaki et al, 2006), which may be necessary for the neuroprotective effects of this medication. Furthermore, a number of animal disease models have found that Li can reduce ischemic damage (Xu et al, 2006). Li-induced increases in the number of astrocytes in rodent hippocampus have been reported (Rocha et al, 1998), as well as enhancement of hippocampus neurogenesis in the dentate gyri of Li-treated mice (Chen et al, 2000). Additionally, Cadotte et al (2003) reported that several weeks of Li treatment inhibited pilocarpine-induced mossy fiber sprouting in rat HC. The ability of Li to attenuate this activation-induced reorganization in the HC supports its role as a neuroprotective agent in an in vivo model that may be relevant to its clinical effects in BD.
We did not find any change in HC size between the AC group and the UM group or healthy comparison group. This small group comprised of only seven patients using valproic acid and patients using lamotrigine, however, and therefore, the lack of a difference between the UM group and those treated with an anticonvulsant must be considered very preliminary. There is one report that valproate-treated patients with BD had larger cingulate gyrus volumes than medication-naïve patients (Atmaca et al, in press) but to our knowledge there are no similar reports of HC volumes in anticonvulsant treated patients. A number of preclinical studies suggest that valproate may share some of the neuroprotective properties that Li appears to possess (Shaltiel et al, 2007; Chen and Manji, 2006), but at least one recent study reported distinct differences between Li and valproate in cultured cerebellar granule cell models, with valproate potentiating cell death in conditions where Li appeared neuroprotective (Jin et al, 2005). Both the preclinical and clinical data supporting a role for valproate and lamotrigine as neuroprotective agents thus require further investigation.
Hh Volume and BD
Our results suggest that the effect of Li is most prominent in the head of the HC. When we divided the HC into its head and body/tail, only this region showed a bilateral volume increase in patients treated with Li compared to unmedicated patients. There have been relatively few MRI studies concerning Hh volume. In these studies, Hh volume has been found to be smaller in patients with post-traumatic stress disorder (Vythilingam et al, 2005), schizophrenia (Csernansky et al, 2002), temporal lobe epilepsy (Bernasconi et al, 2003; Bronen et al, 1995), and age-associated memory impairment (Mega et al, 2002). To our knowledge, our study is the first study in which Hh volume has been measured in patients with BD.
There is evidence suggesting that the neuroanatomic circuit between prefrontal cortex and HC might be involved in the affective symptoms and cognitive deficits associated with BD (Sax et al, 1999). Hippocampal CA1 neurons that project to the medial prefrontal cortex are found predominantly in the Hh in primate models (Barbas and Blatt, 1995; Carmichael and Price, 1995). In one influential model, Mayberg (1997) postulates that the affective and cognitive abnormalities of mood disorders arise from dysregulation in the co-ordinated interaction of HC, prefrontal cortical regions, and anterior cingulated cortex. Our results suggest that the Hh may be particularly responsive to Li treatment and further investigation will be required to specify more precisely the relation between Hh changes and changes in cognitive and affective symptoms following Li treatment.
CONCLUSIONS
Hippocampal volumes did not differ between medication naïve subjects with BD and age and sex-matched healthy comparison subjects. An effect of Li treatment on HC volume was apparent even over a brief treatment period spanning 1–8 weeks. Notably, this increase was apparent in the Hh, but not in the Hbt. It will be of interest for future studies to determine whether there are effects of Li on HC volume over longer periods of treatment (ie months to years) and whether there is a relation between HC volume increases and markers of clinical or cognitive outcome in larger samples of participants.
References
Altshuler LL, Bartzokis G, Grieder T, Curran J, Jimenez T, Leight K et al (2000). An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biol Psychiatry 48: 147–162.
Atmaca M, Ozdemir H, Cetinkaya S, Parmaksiz S, Belli H, Kursad PA et al (in press). Cingulate gyrus volumetry in drug free bipolar patients and patients treated with valproate or valproate and quetiapine. J Psychiatr Res.
Bachmann RF, Schloesser RJ, Gould TD, Manji HK (2005). Mood stabilizers target cellular plasticity and resilience cascades: implications for the development of novel therapeutics. Mol Neurobiol 32: 173–202.
Barbas H, Blatt GJ (1995). Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus 5: 511–533.
Bernasconi N, Bernasconi A, Caramanos Z, Antel SB, Andermann F, Arnold DL (2003). Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain 126: 462–469.
Beyer JL, Kuchibhatla M, Payne ME, Moo-Young M, Cassidy F, Macfall J et al (2004). Hippocampal volume measurement in older adults with bipolar disorder. Am J Geriatr Psychiatry 12: 613–620.
Blumberg HP, Kaufman J, Martin A, Whiteman R, Zhang JH, Gore JC et al (2003). Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry 60: 1201–1208.
Brambilla P, Harenski K, Nicoletti M, Sassi RB, Mallinger AG, Frank E et al (2003). MRI investigation of temporal lobe structures in bipolar patients. J Psychiatr Res 37: 287–295.
Briellmann RS, Syngeniotis A, Jackson GD (2001). Comparison of hippocampal volumetry at 1.5 tesla and 3 tesla. Epilepsia 42: 1021–1024.
Bronen RA, Fulbright RK, Kim JH, Spencer SS, Spencer DD, al-Rodhan NR (1995). Regional distribution of MR findings in hippocampal sclerosis. AJNR Am J Neuroradiol 16: 1193–1200.
Cadotte DW, Xu B, Racine RJ, MacQueen GM, Wang JF, McEwen B et al (2003). Chronic lithium treatment inhibits pilocarpine-induced mossy fiber sprouting in rat hippocampus. Neuropsychopharmacology 28: 1448–1453.
Campbell S, MacQueen GM (2006). An update on regional brain volume differences associated with mood disorders. Curr Opin Psychiatry 19: 25–33.
Campbell S, Marriott M, Nahmias C, MacQueen GM (2004). Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry 161: 598–607.
Carmichael ST, Price JL (1995). Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol 363: 615–641.
Chen BK, Sassi R, Axelson D, Hatch JP, Sanches M, Nicoletti M et al (2004). Cross-sectional study of abnormal amygdala development in adolescents and young adults with bipolar disorder. Biol Psychiatry 56: 399–405.
Chen G, Manji HK (2006). The extracellular signal-regulated kinase pathway: an emerging promising target for mood stabilizers. Curr Opin Psychiatry 19: 313–323.
Chen G, Rajkowska G, Du F, Seraji-Bozorgzad N, Manji HK (2000). Enhancement of hippocampal neurogenesis by lithium. J Neurochem 75: 1729–1734.
Chuang DM (2004). Protective and neurotrophic actions of the mood stabilizer lithium: can it be used to treat neurodegenerative diseases? Crit Rev Neurobiol 16: 83–90.
Chuang DM (2005). The antiapoptotic actions of mood stabilizers: molecular mechanisms and therapeutic potentials. Ann N Y Acad Sci 1053: 195–204.
Csernansky JG, Wang L, Jones D, Rastogi-Cruz D, Posener JA, Heydebrand G et al (2002). Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. Am J Psychiatry 159: 2000–2006.
Dickstein DP, Milham MP, Nugent AC, Drevets WC, Charney DS, Pine DS et al (2005). Frontotemporal alterations in pediatric bipolar disorder: results of a voxel-based morphometry study. Arch Gen Psychiatry 62: 734–741.
First MB, Spitzer RL, Gibbon M, Williams JBW (2001). Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Nonpatient Edition. Biometrics Res. New York State Psychiatr. Institute: New York.
Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN et al (2005). Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry 162: 1256–1265.
Frey BN, Andreazza AC, Cereser KM, Martins MR, Valvassori SS, Reus GZ et al (2006). Effects of mood stabilizers on hippocampus BDNF levels in an animal model of mania. Life Sci 79: 281–286.
Geuze E, Vermetten E, Bremner JD (2005). MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Mol Psychiatry 10: 160–184.
Hashimoto R, Takei N, Shimazu K, Christ L, Lu B, Chuang DM (2002). Lithium induces brain-derived neurotrophic factor and activates TrkB in rodent cortical neurons: an essential step for neuroprotection against glutamate excitotoxicity. Neuropharmacology 43: 1173–1179.
Hauser P, Matochik J, Altshuler LL, Denicoff KD, Conrad A, Li X et al (2000). MRI-based measurements of temporal lobe and ventricular structures in patients with bipolar I and bipolar II disorders. J Affect Disord 60: 25–32.
Jin N, Kovacs AD, Sui Z, Dewhurst S, Maggirwar SB (2005). Opposite effects of lithium and valproic acid on trophic factor deprivation-induced glycogen synthase kinase-3 activation, c-Jun expression and neuronal cell death. Neuropharmacology 48: 576–583.
Judd LL, Akiskal HS, Schettler PJ, Coryell W, Maser J, Rice JA et al (2003). The comparative clinical phenotype and long term longitudinal episode course of bipolar I and II: a clinical spectrum or distinct disorders? J Affect Disord 73: 19–32.
Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA et al (2002). The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry 59: 530–537.
Kim JH, Tien RD, Felsberg GJ, Osumi AK, Lee N (1994). MR measurements of the hippocampus for lateralization of temporal to be epilepsy: value of measurements of the body vs the whole structure. AJR Am J Roentgenol 163: 1453–1457.
Mayberg HS (1997). Limbic-cortical dysregulation: A proposed model of depression. J Neuropsych Clin Neurosci 9: 471–481.
McDonald C, Marshall N, Sham PC, Bullmore ET, Schulze K, Chapple B et al (2006). Regional brain morphometry in patients with schizophrenia or bipolar disorder and their unaffected relatives. Am J Psychiatry 163: 478–487.
McKinnon MC, Svoboda E, Levine B (2007). The frontal lobes and autobiographical memory. In: Miller BL and Cummings JL (eds). The Human Frontal Lobes Functions and Disorders, 2nd edn. The Guilford Press: New York. pp 227–248.
Mega MS, Small GW, Xu ML, Felix J, Manese M, Tran NP et al (2002). Hippocampal atrophy in persons with age-associated memory impairment: volumetry within a common space. Psychosom Med 64: 487–492.
Moore GJ, Bebchuk JM, Hasanat K, Chen G, Seraji-Bozorgzad N, Wilds IB et al (2000b). Lithium increases N-acetyl-aspartate in the human brain: in vivo evidence in support of bcl-2's neurotrophic effects? Biol Psychiatry 48: 1–8.
Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK (2000a). Lithium-induced increase in human brain grey matter. Lancet 356: 1241–1242.
Rajkowska G (2002). Cell pathology in bipolar disorder. Bipolar Disord 4: 105–116.
Rantamaki T, Knuuttila JE, Hokkanen ME, Castren E (2006). The effects of acute and long-term lithium treatments on trkB neurotrophin receptor activation in the mouse hippocampus and anterior cingulate cortex. Neuropharmacology 50: 421–427.
Rocha E, Achaval M, Santos P, Rodnight R (1998). Lithium treatment causes gliosis and modifies the morphology of hippocampal astrocytes in rats. Neuro report 9: 3971–3974.
Sassi RB, Nicoletti M, Brambilla P, Mallinger AG, Frank E, Kupfer DJ et al (2002). Increased gray matter volume in lithium-treated bipolar disorder patients. Neurosci Lett 329: 243–245.
Sax KW, Strakowski SM, Zimmerman ME, DelBello MP, Keck Jr PE, Hawkins JM (1999). Frontosubcortical neuroanatomy and the continuous performance test in mania. Am J Psychiatry 156: 139–141.
Shaltiel G, Chen G, Manji HK (2007). Neurotrophic signaling cascades in the pathophysiology and treatment of bipolar disorder. Curr Opin Pharmacol 7: 22–26.
Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM et al (1999). Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry 56: 254–260.
Strakowski SM, DelBello MP, Zimmerman ME, Getz GE, Mills NP, Ret J et al (2002). Ventricular and periventricular structural volumes in first- versus multiple-episode bipolar disorder. Am J Psychiatry 159: 1841–1847.
Strasser HC, Lilyestrom J, Ashby ER, Honeycutt NA, Schretlen DJ, Pulver AE et al (2005). Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: a pilot study. Biol Psychiatry 57: 633–639.
Swayze II VW, Andreasen NC, Alliger RJ, Yuh WT, Ehrhardt JC (1992). Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biol Psychiatry 31: 221–240.
Velakoulis D, Wood SJ, Wong MT, McGorry PD, Yung A, Phillips L et al (2006). Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry 63: 139–149.
Videbech P, Ravnkilde B (2004). Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry 161: 1957–1966.
Vythilingam M, Luckenbaugh DA, Lam T, Morgan III CA, Lipschitz D, Charney DS et al (2005). Smaller head of the hippocampus in Gulf War-related posttraumatic stress disorder. Psychiatry Res 139: 89–99.
Watson C, Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A et al (1992). Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology 42: 1743–1750.
Xu XH, Zhang HL, Han R, Gu ZL, Qin ZH (2006). Enhancement of neuroprotection and heat shock protein induction by combined prostaglandin A1 and lithium in rodent models of focal ischemia. Brain Res 1102: 154–162.
Acknowledgements
We are grateful to the patients and their families for their assistance. We thank Cathy Preete and Benjamin Doxtdator for their assistance in preparation of this manuscript and Laura Garrick, Helen Begin, Cindy D'Amico, Scott Simmons, and Tana Pati for assistance with patient scheduling. We are also grateful to Geoff Hall and Andrea Milne for assistance in running the brain imaging protocol and Marcella Rincon Castro for assistance in the imaging analysis. This study was supported by the Canadian Institutes of Health Research and the Ontario Mental Health Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yucel, K., Taylor, V., McKinnon, M. et al. Bilateral Hippocampal Volume Increase in Patients with Bipolar Disorder and Short-term Lithium Treatment. Neuropsychopharmacol 33, 361–367 (2008). https://doi.org/10.1038/sj.npp.1301405
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301405
Keywords
This article is cited by
-
Comparison of lithium levels between suicide and non-suicide fatalities: Cross-sectional study
Translational Psychiatry (2022)
-
Lithium as a Neuroprotective Agent for Bipolar Disorder: An Overview
Cellular and Molecular Neurobiology (2022)
-
Hippocampal tail volume as a predictive biomarker of antidepressant treatment outcomes in patients with major depressive disorder: a CAN-BIND report
Neuropsychopharmacology (2020)
-
Cortical thickness and subcortical volumes alterations in euthymic bipolar I patients treated with different mood stabilizers
Brain Imaging and Behavior (2019)
-
Progression from selective to general involvement of hippocampal subfields in schizophrenia
Molecular Psychiatry (2017)