Abstract
The cannabinoid CB1 receptor is one of the most abundant G protein-coupled receptors in the brain and is a promising target of therapeutic drug development. Success of drug development for neuropsychiatric indications is significantly enhanced with the ability to directly measure spatial and temporal binding of compounds to receptors in central compartments. We assessed the utility of a new positron emission tomography (PET) radioligand to image CB1 receptors in monkey brain. [11C]MePPEP ((3R,5R)-5-(3-methoxy-phenyl)-3-((R)-1-phenyl-ethylamino)-1-(4-trifluoromethyl-phenyl)-pyrrolidin-2-one) has high CB1 affinity (Kb=0.574±0.207 nM) but also moderately high lipophilicity (measured LogD7.4=4.8). After intravenous injection of [11C]MePPEP, brain activity reached high levels of almost 600% standardized uptake value (SUV) within 10–20 min. The regional uptake was consistent with the distribution of CB1 receptors, with high radioactivity in striatum and cerebellum and low in thalamus and pons. Injection of pharmacological doses of CB1-selective agents confirmed that the tracer doses of [11C]MePPEP reversibly labeled CB1 receptors. Preblockade or displacement with two CB1 selective agents (ISPB; (4-(3-cyclopentyl-indole-1-sulfonyl)-N-(tetrahydro-pyran-4-ylmethyl)-benzamide) and rimonabant) showed that the majority (>89%) of brain uptake in regions with high receptor densities was specific and reversibly bound to CB1 receptors in the high binding regions. [11C]MePPEP was rapidly removed from arterial plasma. Regional brain uptake could be quantified as distribution volume relative to the concentration of parent radiotracer in plasma. The P-glycoprotein (P-gp) inhibitor DCPQ ((R)-4-[(1a,6,10b)-1,1-dichloro-1,1a,6,10b-tetrahydrodibenzo[a,e]cyclopropa[c]cyclohepten-6-yl]-[(5-quinolinyloxy)methyl]-1-piperazineethanol) did not significantly increase brain uptake of [11C]MePPEP, suggesting it is not a substrate for this efflux transporter at the blood–brain barrier. [11C]MePPEP is a radioligand with high brain uptake, high specific signal to CB1 receptors, and adequately fast washout from brain that allows quantification with 11C (half-life=20 min). These promising results in monkey justify studying this radioligand in human subjects.
Similar content being viewed by others
INTRODUCTION
Of the first two described cannabinoid receptor subtypes, the CB1 receptor is found mainly in the central nervous system, and the CB2 receptor is found mainly in peripheral immune system cells (Howlett et al, 2002). CB1 receptors are especially abundant in cerebellum, substantia nigra, globus pallidus, hippocampus, and lateral striatum (Herkenham et al, 1990). The CB1 receptor mediates several pharmacological effects and is an active target for therapeutic drug development (Howlett et al, 2002; Le Foll and Goldberg, 2005). Cannabinoid receptor ligands may be useful for the treatment of pain (Walker et al, 1999), nausea (Simoneau et al, 2001), appetite control (Gelfand and Cannon, 2006; Kirkham and Tucci, 2006), and smoking cessation (Gelfand and Cannon, 2006).
The development of an in vivo imaging probe for the CB1 receptor has been difficult and largely unsuccessful. Similar to the active principal ingredient in marijuana, Δ9-tetrahydrocannabinol, most of the proposed radioligands are highly lipophilic. This physiochemical property commonly causes two deficiencies of radioligands. First, plasma protein binding tends to be high (>99%) so that only a small proportion (<1%) is available to cross the blood–brain barrier. Second, brain has a high percentage of lipids, which comprise ∼60% of its dry weight (Bennett and Horrobin, 2000). Thus, the majority of a lipophilic tracer that is able to cross the blood–brain barrier is often bound nonspecifically in the brain's abundant lipid environment. Thus, tracers that are too lipophilic tend to have low brain uptake and low ratios of specific to nonspecific binding. These two deficiencies apply to most radiotracers developed to date for the CB1 receptor. These ligands have been based on the structures of rimonabant (Cosenza et al, 2000; Gatley et al, 1996; Horti et al, 2006; Katoch-Rouse et al, 2003; Mathews et al, 2000, 2002), cannabinoids (Charalambous et al, 1991), and alkylindoles (Gifford et al, 2002; Tamagan et al, 1999). In contrast to these older agents, 11C-JHU75528 is a notable exception (see ‘Discussion’) and has moderate brain uptake and specific signal (Horti et al, 2006).
To correct the deficiencies of most previous CB1 receptor ligands, we recently developed [11C]MePPEP ((3R,5R)-5-(3-methoxy-phenyl)-3-((R)-1-phenyl-ethylamino)-1-(4-trifluoromethyl-phenyl)-pyrrolidin-2-one), which is a CB1 mixed inverse agonist and antagonist (Figure 1). It has moderately high lipophilicity (measured LogD7.4=4.8) but still maintains high affinity and selectivity for the CB1 receptor (Kb at human CB1=0.574±0.207 nM compared to CB2=363±87.4 nM; see ‘Results’). The primary purposes of this study were to carefully examine the kinetics of [11C]MePPEP uptake in nonhuman primate brain and to determine whether human studies would be justified. Specifically, we assessed the pharmacological specificity of brain uptake and quantified the imaging data with compartmental modeling and serial arterial measurements of parent radiotracer separated from radiometabolites.
MATERIALS AND METHODS
[γ-35S]GTP Binding Assay Methods
[γ-35S]GTP binding was determined in a 96-well-plate format using a modification of the antibody capture scintillation proximity technique previously described (DeLapp et al, 1999). Briefly, 100 μl (0.9 units/well) of membrane preparation from Sf9 cells that ectopically express human CB1 or CB2 cannabinoid receptors (PerkinElmer Life and Analytical Sciences, Boston, MA) were incubated for 15 min with 50 μl of test compound diluted in 0.5% fatty acid-free bovine serum albumin (BSA) (Serologicals Corp., Norcross, GA). GDP was added to CB1 (1 μM final concentration) and CB2 (0.05 μM final concentration) receptor membranes before incubation. Antagonist/inverse agonist curves were generated in the presence of an EC80 concentration of methanandamide and 0.5% BSA. Following the incubation period, 50 μl [γ-35S]GTP (500 pM final concentration; PerkinElmer Life and Analytical Sciences, Boston, MA) were added to each well and incubated for 35 min. The labeled membranes were solubilized with 0.27% Nonidet P40 for 30 min followed by 30 min of incubation with 20 μl (final dilution of 1 : 300) of rabbit polyclonal Gαi-3 antibody (Covance, Princeton, NJ). Following the antibody incubation, 50 μl (1.25 mg/well) anti-rabbit scintillation proximity assay beads (GE Healthcare, Piscataway, NJ) were added per well and the plates incubated for an additional 60 min. The plates were centrifuged for 10 min at 183g and counted for 1 min per well using a Wallac MicroBeta TriLux scintillation counter (PerkinElmer, Boston, MA). All incubations took place at room temperature in GTP-binding assay buffer (20 mM HEPES, 100 mM NaCl, 5 mM MgC12, pH 7.4).
Data were analyzed using a four-parameter logistic-reduced fit with Marquardt–Levenberg algorithm and 200 iterations using Activity Base and XLFit2 (IDBS, Emeryville, CA). Background was subtracted from all wells, data were normalized to a full agonist response (1 μM methanandamide), and efficacy was expressed as a percentage of 1 μM methanandamide. Antagonist/inverse agonist percent inhibition data were normalized to results generated with an EC80 concentration of methanandamide. Kb values were determined using a modification of the Cheng–Prusoff relationship: Kb=IC50/(1+(agonist)/EC50) where IC50 is determined from a four parameter fit of displacement curves, [agonist]=EC80 of full agonist (methanandamide), and EC50 is determined from a four-parameter fit of the full agonist concentration response curve (Cheng and Prusoff, 1973). Mean EC50 and percent inhibition values were calculated as a mean of three independent determinations±standard error of the mean (SEM).
Broad screening of common cell surface targets was performed by standard radioligand binding techniques through a contract agreement with Cerep (Paris, France). Competitive binding was either not significant or had a Ki value of >10 μM at the following receptor, ion channel, or enzyme targets: acetylcholinesterase, CCK1, CCK2, EDG-2, Cox1, Cox2, adenosine A1, adenosine A3, adrenergic alpha1, adrenergic alpha2, angiotensin AT1, adrenergic β1, adrenergic β2, benzodiazepine site, Ca2+ channel (verapamil site), dopamine D1, dopamine D2S, GABA, Ghrelin, histamine H1, histamine H2, K+ channel (HERG), nonselective muscarinic, NE transporter, NK1, NK2, nonselective opiate, nonselective 5-HT.
Preparation and Analysis of [11C]MePPEP
[11C]Iodomethane produced by the GE PETtrace Microlab was delivered with helium carrier gas at a flow rate of 10 ml/min into a 2 ml stainless loop containing 1 mg phenolic precursor (22 nM) and 1 equivalent tetrabutylammonium hydroxide in 80 μl dimethyl formamide. The reaction proceeded for 5 min, and the entire contents of the loop were injected onto the HPLC column. The labeled product was purified on C18 column (Phenomenex luna, 10 × 250 mm) with isocratic mobile phase (70 : 30=acetonitrile/0.1 M ammonium formate, pH adjusted to 7.5 with 1 M ammonium hydroxide in water) at the flow rate of 6 ml/min. Product eluted at 12 min and precursor at 6.3 min. Solvents were removed by rotary evaporation at 80°C. The product was formulated with 10 ml saline containing 20 mg Tween 80 within 35 min of the end of bombardment.
Measurement of LogD7.4
[11C]MePPEP was dissolved in 0.9% NaCl/10% ethanol to a specific concentration of about 10 μCi/μl. The radiochemical purity of this preparation was greater than 99%. Six determinations of LogD7.4 were carried out at room temperature in n-octanol and 0.15 M phosphate buffer (pH 7.4), as previously described (Zoghbi et al, 1993), but with correction of the radioactivity associated with parent radioligand in the aqueous phase after radio-HPLC. The percent error of the γ-countermeasurements of the samples with the least counts was 4.6±0.3%.
Animals
All animal experiments complied with the Guide for Care and Use of Laboratory Animals and were approved by the NIMH Animal Care and Use Committee. We used six male rhesus monkeys (weight: 12.6±2.0 kg, with these and subsequent data expressed as mean±SD), four of which underwent arterial blood sampling. Animals were immobilized with ketamine (10 mg/kg i.m.) for intubation. After transportation to the positron emission tomography (PET) suite, the animals were placed under isoflurane anesthesia (1–2%), and the head was immobilized in a stereotactic frame. To minimize the effects of ketamine, scans started at least 90 min after ketamine administration.
We used the high resolution research tomograph (HRRT; Siemens/CPS, Knoxville, TN, USA), which has a reconstructed resolution of 2.5 mm at full-width half-maximum in all directions in 3D mode. A transmission scan was initially performed for attenuation correction. [11C]MePPEP was administered over ∼60 s. Scans of 120 min (33 frames) were acquired in all studies, whereas longer scans (39 frames over 150 min) were acquired in one monkey to evaluate the effect of the scan duration on the plasma compartmental analysis.
Arterial blood was collected in heparin-treated syringes from four monkeys at 15, 30, 45, 60, 75, 90, 105 s and 2, 3, 5, 10, 30, 60, 75, 90, 120 min after injection. The parent tracer, separated from radiometabolites, was measured as previously described (Zoghbi et al, 2005). We also measured the plasma-free fraction (f1) of [11C]MePPEP in two monkeys (E and F; Table 1). Plasma-free fraction was measured by ultrafiltration through Centrifree membrane filters (Amicon Division, W.R. Grace and Co., Danvers, MA, USA) (Gandelman et al, 1994) in two monkeys. Adequate radioactivity was used so that the percentage of counting error in the least radioactive samples ranged between 3.0±0.3 and 5.3±1.3% at one standard deviation.
Receptor preblocking studies were performed in two monkeys using rimonabant (previously called SR141716A) and ISPB (4-(3-cyclopentyl-indole-1-sulfonyl)-N-(tetrahydro-pyran-4-ylmethyl)-benzamide) (Figure 1), both of which are selective for the CB1 receptor and have mixed inverse agonist and antagonist properties. ISPB (1.0 mg/kg i.v.) or rimonabant (3.0 mg/kg i.v.) were injected 10 min before [11C]MePPEP (Table 1, monkeys A and B). A displacement study was also carried out for one monkey, in which nonradioactive ISPB (1.5 mg/kg i.v.) was injected 60 min after [11C]MePPEP (Table 1, monkey A).
In this displacement study, data of the initial 9 min were not acquired because of a scanner problem. These studies of preblocking were performed in the afternoon, following the baseline scan of the same animal in the morning.
Each monkey had a T1-weighted magnetic resonance imaging (MRI) scan on a GE Sigma 1.5 T scanner (SPGR, TR/TE/flip angle=13.1/5.8 ms/45°, 0.4 × 0.4 × 1.5 mm with coronal acquisitions on a 256 × 256 × 60 matrix).
Image Analysis
Regional brain radioactivity was obtained with a modified template-based method that consisted of three steps (Yasuno et al, 2002). The first step involved the spatial transformation of the regional template from a model MRI to an individual animal's MRI. The second step was the manual correction of the error of transformed volumes of interest with reference to the animal's MRI (Figure 2). Finally, corrected template volumes were linearly transformed to the PET images using the parameters obtained from the coregistration of the MRI. Seven template volumes were defined: cerebellum (2.1 cm3), prefrontal cortex (8.8 cm3), lateral temporal cortex (5.9 cm3), medial temporal region including the hippocampus (3.1 cm3), striatum (2.7 cm3), thalamus (0.9 cm3), and pons (0.8 cm3).
Estimation of Distribution Volume with Arterial Input Function
The brain and plasma data were analyzed with one- and two-tissue compartment models using rate constants (K1, k2, k3, and k4) as previously described (Laruelle et al, 1994). Total distribution volume VT measures specific receptor binding, nonspecific binding, and free radiotracer. The nondisplaceable component VND is the sum of the latter two—nonspecific binding plus free radiotracer.
For the one-tissue compartment model:

For the two-compartment model, VT is the sum of the distribution volumes of nondisplaceable uptake (VND) and specific binding (VS).

The kinetic analyses were performed using the software program PMOD 2.75 (Burger et al, 1998).
Time-Stability of Parameter Estimates
The relationship between parameter estimates and the duration of the scan was analyzed in one monkey with increasingly truncated data sets from 0–150 to 0–60 min (Table 1, monkey A). The estimated parameters were compared with the reference values obtained with the complete 150 min data set. For the time activity data from the mean of all measured regions, specific binding was estimated in the two-tissue compartmental model. The solution was considered stable after time t if the result was within 10% of that from the analysis of the entire data set (ie 0–150 min).
Effect of P-gp on Brain Uptake and Peripheral Disposition of [11C]MePPEP
We compared the uptake of [11C]MePPEP in three studies (Table 1, monkey D, E, and F) with and without the administration of 8 mg/kg i.v. of the P-glycoprotein (P-gp) inhibitor DCPQ ((R)-4-[(1a,6,10b)-1,1-dichloro-1,1a,6,10b-tetrahydrodibenzo[a,e]cyclopropa[c]cyclohepten-6-yl]-[(5-quinolinyloxy)methyl]-1-piperazineethanol; Figure 1). DCPQ was previously reported as compound 14b (Pfister et al, 1995).
Under terms of the Material Transfer Agreement with Xenova Group, Ltd (UK) we could not use their better known P-gp inhibitor tariquidar, previously called XR9576 (Roe et al, 1999) with proprietary compounds in the current collaboration. Instead, we compared the efficacy of tariquidar and DCPQ to enhance brain uptake of [11C]lopramide, which is an avid substrate for P-gp (Sadeque et al, 2000). DCPQ and tariquidar (each at 8 mg/kg i.v.) was administered 30 min before [11C]loperamide and increased rhesus brain activity to 415 and 316%, respectively.
Statistical Analysis
Goodness-of-fit by nonlinear least-squares analysis was evaluated using the model selection criterion (MSC), which is a modification of the Akaike information criterion (Akaike, 1974). MSC gives greater values for better fitting. Goodness-of-fit by one- and two-tissue compartmental models was compared with F statistics (Hawkins et al, 1986).
The standard error of nonlinear least-squares estimation for rate constants was given by the diagonal of the covariance matrix (Carson, 1986) and expressed as a percentage of the rate constants (coefficient of variation, %COV). In addition, %COV of total distribution volume was calculated from the covariance matrix using the generalized form of error propagation equation (Bevington and Robinson, 2003), where correlations among parameters were taken into account.
RESULTS
In Vitro Activity of MePPEP
In membranes prepared from Sf9 cells ectopically expressing the human CB1 or CB2 receptors, in vitro functional binding activity of nonlabeled MePPEP was determined using an antibody capture scintillation proximity [γ-35S]GTP binding assay and compared to rimonabant (previously called SR141716A). MePPEP inhibited functional [γ-35S]GTP binding at the human CB1 receptor with high potency (Kb=0.574±0.207 nM) compared to rimonabant (Kb=5.96±0.209 nM). MePPEP was significantly less potent at the human CB2 receptor (Kb=363±77.9 nM for MePPEP and Kb>10 000 nM for rimonabant at CB2).
Preparation of [11C]MePPEP
The radioligand was prepared within 35 min of the end of bombardment and had yield=2.5±1.1%, radiochemical purity=100±0%, chemical purity=99.9±0%, and specific activity at time of injection=78.1±54.9 GBq/μmol (n=12 batches). The mean injected activity was 232±70 MBq.
The LogD7.4 of [11C]MePPEP was 4.8±0.3 (n=6), measured in octanol/saline at pH 7.4.
Pharmacological Effects in Nonhuman Primates
In PET scans, the injected mass dose of [11C]MePPEP was 0.11±0.06 μg/kg. In all baseline and blocking experiments using ISPB (1.0–1.5 mg/kg) and rimonabant (3.0 mg/kg), the differences between pre- and postinjection vital signs were: <17 mm Hg for systemic blood pressure, <17 min−1 for pulse, <3 min−1 for respiratory rate, and <0.1°C for temperature.
Brain Uptake of [11C]MePPEP
Following injection of [11C]MePPEP, brain activity increased to high levels (almost 600% standardized uptake (SUV) in cerebellum), with peak values within 10–20 min. The distribution of activity was consistent with binding to CB1 receptors, with high levels in striatum and cerebellum and low in thalamus and pons (Figures 3 and 4a). The brain uptake of [11C]MePPEP was preblocked with receptor saturating doses of nonradioactive ISPB (1.0 mg/kg i.v.) or rimonabant (3.0 mg/kg i.v.) (Figure 4b). In addition, brain uptake was rapidly displaced by nonradioactive ISPB (1.5 mg/kg i.v.) at 60 min after tracer injection (Figure 4c). In summary, brain uptake of [11C]MePPEP was consistent with reversible labeling of CB1 receptors, showing an appropriate regional distribution, pharmacological selectivity, and saturability.
PET summation image in rhesus brain. PET images (middle column) were summed from 60 to 120 min. The corresponding MR images are on left column, and the fused PET and MR images are on right column. Upper row: slice including cerebellum and medial and lateral temporal cortex. Middle row: slice including striatum, thalamus, and lateral temporal cortex. Lower row: slice including prefrontal cortex.
(a) Time course of regional brain activities after injection of [11C]MePPEP. Activity is shown as %SUV which normalizes for injected dose and body weight. %SUV=(% injected activity/cm3 tissue)(g body weight) cerebellum, ▵; striatum, × ; prefrontal cortex, ◊; lateral temporal cortex, □; medial temporal region, ○; thalamus, +; and pons, ▴. (b) Receptors were blocked by pretreatment with ISPB (1.0 mg/kg i.v.) before injection of [11C]MePPEP. (c) Radioligand was displaced by ISPB (1.5 mg/kg i.v.) injected 60 min after [11C]MePPEP. Data from the initial 9 min are missing because of scanner malfunction.
Arterial Plasma Analysis
The radioligand was quickly metabolized and represented 83±12, 53±17, 21±7, and 14±5% of total plasma activity at 5, 10, 30, and 60 min, respectively, in all the four monkey studies. [11C]MePPEP was rapidly removed from arterial plasma, with a clearance rate of 361±40 ml/min. Plasma activity of unchanged [11C]MePPEP peaked at ∼1 min and decreased to 3.1±0.9% of the peak by 10 min.
Monkey arterial plasma samples had four radioactive peaks. A sample radiochromatogram of plasma at 10 min (Figure 5a) was composed of polar peak 1 (34.4%); two intermediate peaks, 2 (5.4%) and 3 (1.2%); and parent radioligand 4 (59%). The plasma parent composition declined rapidly to about 18% as early as 30 min after the injection of [11C]MePPEP. Radiometabolite 1 was the major radioactive component at the end of the study (Figure 5b).
(a) Radiochromatogram of a plasma sample obtained at 10 min after intravenous injection of [11C]MePPEP. Peak 1 was the most polar radiometabolite and eluted in the void volume of the column. Peak 4 was the parent radioligand. (b) Representative plasma composition over time of [11C]MePPEP (4) and its three radiometabolites (1, 2, and 3).
Nonlinear Least-Squares Compartmental Analyses
We used brain activity and serial arterial plasma measurements of parent radiotracer to estimate distribution volume in brain using four scanning sessions in four different animals (monkeys A–D; Table 1). Convergence was achieved in the baseline scans using both one- and two-tissue compartmental model analyses of 120 min scan data, but the two-tissue compartmental model provided significantly better fits (Tables 2 and 3; Figure 6). MSC values were higher for two-tissue (3.4±0.7) than for one-tissue (2.2±0.7) compartmental models, and this difference was significant by F-test in all regions of all animals (p<0.001).
One- and two-tissue compartment analysis. Fitting by two-tissue compartmental model (solid line) was significantly better that that by one-tissue compartmental model (dashed line) for measured %SUV of all regions. The %SUV of all regions was calculated from the volume-weighted average of cerebellum, striatum, prefrontal cortex, lateral temporal cortex, medial temporal region, and pons.
In two-tissue compartmental model, the standard errors of individual rate constants showed poor identifiability with COV values>10% in most regions. However, VS and VT were relatively well identified in each model, with COV values <8%. Although values of VT obtained with the one- and two-tissue compartment models correlated well (r=0.99), the one-tissue model apparently underestimated VT by an average of 12% (range 426%), which may have been caused by its relatively poor goodness of fit.
We also calculated distribution volume in two scanning sessions (monkeys A and B, Table 1) with preblockade of rimonabant or ISPB and compared them to the baseline VT of same animals. In these two studies, the mean regional value of nondisplaceable uptake (VND) in high binding regions (ie all except thalamus and pons) was about 11% of VT (Table 4). Thus, we estimate that 89% of total brain uptake was associated with CB1 receptors.
Effect of P-gp on Brain Uptake and Peripheral Disposition of [11C]MePPEP
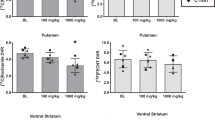
DCPQ (8 mg/kg i.v.) significantly increased the uptake of [11C]MePPEP in all brain regions (Figure 7a). In contrast, DCPQ had no significant effect on the clearance of [11C]MePPEP measured in arterial plasma corrected for radiometabolites (Figure 7b). In the three animals tested, DCPQ caused a ∼17% increase of the mean area under the brain curves of all six regions, calculated from 0 to 120 min (Table 5). These results showed an apparent effect of P-gp blockade—increased brain uptake in the absence of noticeable changes of total tracer concentration in plasma. However, we also examined the effect of DCPQ on f1 of tracer. DCPQ increased f1 in each animal, with a mean increase of 10% (Table 5).
Effect of DCPQ on brain uptake and peripheral clearance of [11C]MePPEP. (a) Average uptake of all measured regions of all animals. DCPQ (8 mg/kg i.v.) increased uptake in all regions. Open circles represent baseline and solid circles DCPQ results. (b) [11C]MePPEP plasma radioactivity, corrected for metabolites, are shown for baseline (○) and P-gp inhibitor ( × ) studies. Values are total concentration of plasma [11C]MePPEP—free plus protein bound. Left curve shows values from 0 to 10 min; right curve from 5 to 120 min. The curves suggest no significant effect of P-gp inhibition on peripheral clearance.
Correction of the area under the curve for change in f1 suggested that DCPQ caused only a 6% effect on brain activity independent of its peripheral effects. That is, the corrected ratio under the brain activity curves between baseline and DCPQ was 1.06 (=1.17/1.10). An increase of 6% with P-gp blockade is quite small relative to tracers that are known to be efflux pump substrates, which are elevated 400–1300% with genetic inactivation of P-gp (Schinkel et al, 1996). This 6% increase may also be insignificant in light of possible measurement errors for the small absolute values of f1 (0.04–0.07%; Table 5).
DISCUSSION
The uptake of [11C]MePPEP in nonhuman primate brain demonstrated reversible labeling of CB1 receptors. Brain uptake showed appropriate pharmacological properties, as it could be displaced and/or blocked by agents from two different chemical classes, ISPB and rimonabant. The clearance of [11C]MePPEP in monkey was adequately fast to generate peak brain uptake within 20 min, which is well within the effective imaging time of 11C that has a half-life of 20 min.
The P-gp inhibitor DCPQ (8 mg/kg i.v.) increased brain uptake by ∼17%, with insignificant change in the parent tracer in plasma (ie the amount of tracer exposed to brain; Figure 7). However, DCPQ increased f1 by ∼10%, and the corrected ratio of area under the curve between baseline and DCPQ was 1.06 (=1.17/1.10), which may merely reflect errors in measurement of the low values of f1 (Table 5). In addition, a 17% increase is small compared to >400% found for agents that are avid substrates for P-gp (Schinkel et al, 1996). Thus, our data suggest that [11C]MePPEP is a relatively insignificant substrate for P-gp in monkey brain.
Advantages of [11C]MePPEP
What are the relative advantages and limitations of [11C]MePPEP as a probe for central CB1 receptors? The advantages include:
-
1)
[11C]MePPEP has high brain uptake, with peak levels of almost 600% SUV. Such high uptake means that brain activity can be more accurately quantified and/or the injected activity can be reduced. For comparison, the peak uptake of two other PET radiotracers in monkey is 300% SUV in striatum for the dopamine D2/3 receptor probe [11C]raclopride (Nyberg et al, 2002) and 500% SUV in medial temporal region for the 5-HT1A receptor probe [11C](R)-(−)-RWAY (Yasuno et al, 2006). The high brain uptake of [11C]MePPEP reflected, in part, CB1 receptor density, which is the highest of any G protein-coupled receptor in brain (Begg et al, 2005). The density (Bmax) of CB1 receptors in cerebellum in rat is 1752 fmol/mg protein (Hirst et al, 1996), compared to dopamine D2 receptors in striatum (Bmax=247 fmol/mg protein) (Dewar et al, 1989) and 5-HT1A receptors in hippocampal region (312 fmol/mg protein) (Khawaja et al, 1995).
-
2)
[11C]MePPEP has a high percentage of specific binding. We performed compartmental analysis in two different preblocked conditions. The results suggested at least 89% of uptake was specific in high binding regions. If we had administered a dose that completely blocked CB1 receptors, then the estimate of specific uptake would have been even higher. In summary, both baseline and preblocked analysis showed that the vast majority (>89%) of brain uptake is bound to CB1 receptors (ie specific binding).
-
3)
The rapid plasma clearance of [11C]MePPEP is important to measure distribution volume. As a general rule, quantitation of brain uptake requires imaging before and slightly after the time of peak uptake. Receptor density and plasma clearance have opposite effects on the time of peak uptake. That is, higher densities of receptors cause later times of peak uptake, as these regions can bind more ligand at equilibrium. In contrast, faster plasma clearance causes an earlier peak in all organs, because the ligand is removed from the body more rapidly. As CB1 receptors are present at such high densities in brain, the clearance of a useful radioligand must be rapid so that the peak can occur within the useful imaging time of the radionuclide. Thus, the rapid plasma clearance of [11C]MePPEP (see ‘Results’) is critical to quantify distribution volume, which is proportional to receptor density.
-
4)
[11C]MePPEP is relatively easily prepared with high purity (>99%) and in reasonable overall yield (3%) using a commercially available 11C-methylation apparatus.
-
5)
The short half-life of 11C allows two or more scanning studies to be completed in a single subject in 1 day. Such a repeated scanning schedule can accommodate pharmacological challenges or measurements of receptor occupancy by CB1 receptor-selective medications.
Limitation of [11C]MePPEP
The limitations of [11C]MePPEP and imaging the CB1 receptor in general include:
-
1)
The plasma-free fraction f1 of [11C]MePPEP was quite low (0.04–0.07%) and difficult to measure accurately. Brain uptake is proportional to f1, as protein-bound drug cannot cross the blood–brain barrier. Thus, relatively small changes in protein binding would have significant effects on total brain uptake. For example, if protein binding decreases from 99.9 to 99.8%, then f1 and brain uptake would increase 100%, from 0.1 to 0.2%. Intersubject variability of baseline distribution volumes in our studies may not reflect actual differences in receptor densities. Instead, it may reflect biological variability in f1 or our limited accuracy to measure such low values. Future studies should carefully assess methods to accurately measure and assess the impact of f1 on brain uptake of [11C]MePPEP.
-
2)
Although not a limitation specific to [11C]MePPEP, the monkey brain has no region that could be used as reference tissue. We quantified brain uptake relative to concentrations of parent radiotracer in plasma. Reference tissue methods compare uptake in receptor-rich to receptor-free regions within brain and are much simpler to perform. Even the pons, which has a relatively low density of CB1 receptors, showed significant displacement and blockade (Figure 4b and c). Some of this displaceable activity derived from surrounding regions, because of the limited resolution of the PET camera and the resulting spillover of activity from adjacent tissue. The human brain may be large enough so that a small area like pons could be used as a reference region. Analysis with a reference region would not only avoid arterial plasma sampling but also obviate the need to measure f1. That is, normalizing to a reference region effectively corrects for intersubject variations in f1.
-
3)
[11C]MePPEP may generate radiometabolite(s) that enter monkey brain. The vast majority of brain activity was displaced or preblocked, suggesting that any putative metabolite must retain significant receptor affinity. Nevertheless, additional studies in rodents would be useful to detect the presence and the level of radiometabolites extracted from brain, and any future human studies with [11C]MePPEP should carefully examine the time stability of distribution volume measurements.
-
4)
A major concern about [11C]MePPEP is whether humans will have slower brain washout such that distribution volume measurements are unstable within the practicable imaging time of 11C. Scans typically span a total 60–150 min, depending on injected activity and sensitivity of the camera. Brain washout is highly dependent on the peripheral clearance of the radiopharmaceutical, with faster peripheral clearance causing fasting brain washout. In the absence of pharmacokinetic data on MePPEP, human studies with tracer doses of [11C]MePPEP are the most expeditious method to determine the utility of the radiotracer. Because of its overall favorable performance in monkeys, we plan to study [11C]MePPEP in humans.
Comparison with Other CB1 Receptor Radioligands
11C-JHU75528 is an analog of rimonabant and was recently reported as an effective PET probe for CB1 receptors (Horti et al, 2006). As mentioned in the ‘Introduction’, it appears to be the best radioligand published to date for in vivo imaging of the CB1 receptor. 11C-JHU75528 and [11C]MePPEP had similar distribution and time of peak uptake (∼20 min) in baboon and rhesus brain, respectively. However, the peak uptake of 11C-JHU75528 may be almost sixfold lower than that of [11C]MePPEP. The brain peak uptake of 11C-JHU75528 is reported to be 0.4% injected activity per 100 ml in putamen in male baboon (26 kg), which corresponds to an SUV of 104%. In contrast, [11C]MePPEP showed peak striatal uptake of ∼600% SUV in our study. The percentage-specific binding of these two tracers is difficult to compare, because of different measurements in the two studies. Nevertheless, preblockade decreased striatal uptake of 11C-JHU75528 by about 50%, which is similar to our results with [11C]MePPEP (Figure 4a and b). Note that these comparisons of brain time activity curves do not correct for changes in arterial input function, which are required for accurate quantitation of the percentage-specific signal in brain. In brief, [11C]MePPEP and 11C-JHU75528 appear to have fairly similar kinetics of brain uptake and may have similar percentage of specific signal. However, the peak uptake of [11C]MePPEP is about sixfold greater than that of 11C-JHU75528. Both 11C-JHU75528 and [11C]MePPEP are likely to be assessed in humans. Because these two ligands have different structures, species differences in clearance, metabolism, and plasma protein binding may make one tracer clearly superior to the other for human studies.
Conclusion
[11C]MePPEP is promising tracer to measure CB1 receptors. It readily entered monkey brain, had peak levels within 20 min, and showed relatively stable measurements of distribution volume within 90 min. For quantitation of distribution volumes using an arterial input, the two-tissue compartment analysis was superior to one-tissue for all measured regions. These promising results justify trials of [11C]MePPEP in humans, for which brain uptake should be carefully analyzed for measuring distribution volume parameters and to provide any evidence of radiometabolites.
References
Akaike H (1974). A new look at the statistical model identification. IEEE Trans Automat Control AC 19: 716–723.
Begg M, Pacher P, Batkai S, Osei-Hyiaman D, Offertaler L, Mo FM et al (2005). Evidence for novel cannabinoid receptors. Pharmacol Ther 106: 133–145.
Bennett CN, Horrobin DF (2000). Gene targets related to phospholipid and fatty acid metabolism in schizophrenia and other psychiatric disorders: an update. Prostaglandins Leukot Essent Fatty Acids 63: 47–59.
Bevington PR, Robinson DK (2003). Data Reduction and Error Analysis for the Physical Sciences. McGraw-Hill: New York.
Burger C, Mikolajczyk K, Grodzki M, Rudnicki P, Szabatin M, Buck A (1998). JAVA tools quantitative post-processing of brain PET data. J Nucl Med 39: 277P.
Carson RE (1986). Parameter estimation in positron emission tomography. In: Phelps ME, Mazziotta JC, Schelbert HR (eds). Positron Emission Tomography and Autoradiography: Principles and Applications for the Brain and Heart. Raven Press: New York. pp 347–390.
Charalambous A, Marciniak G, Shiue CY, Dewey SL, Schlyer DJ, Wolf AP et al (1991). PET studies in the primate brain and biodistribution in mice using (−)-5′-18F-delta 8-THC. Pharmacol Biochem Behav 40: 503–507.
Cheng Y, Prusoff WH (1973). Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22: 3099–3108.
Cosenza M, Gifford AN, Gatley SJ, Pyatt B, Liu Q, Makriyannis A et al (2000). Locomotor activity and occupancy of brain cannabinoid CB1 receptors by the antagonist/inverse agonist AM281. Synapse 38: 477–482.
DeLapp NW, McKinzie JH, Sawyer BD, Vandergriff A, Falcone J, McClure D et al (1999). Determination of [35S]guanosine-5′-O-(3-thio)triphosphate binding mediated by cholinergic muscarinic receptors in membranes from Chinese hamster ovary cells and rat striatum using an anti-G protein scintillation proximity assay. J Pharmacol Exp Ther 289: 946–955.
Dewar KM, Montreuil B, Grondin L, Reader TA (1989). Dopamine D2 receptors labeled with [3H]raclopride in rat and rabbit brains. Equilibrium binding, kinetics, distribution and selectivity. J Pharmacol Exp Ther 250: 696–706.
Gandelman MS, Baldwin RM, Zoghbi SS, Zea-Ponce Y, Innis RB (1994). Evaluation of ultrafiltration for the free fraction determination of single photon emission computed tomography (SPECT) tracers: β-CIT, IBF, and iomazenil. J Pharm Sci 83: 1014–1019.
Gatley SJ, Gifford AN, Volkow ND, Lan R, Makriyannis A (1996). 123I-labeled AM251: a radioiodinated ligand which binds in vivo to mouse brain cannabinoid CB1 receptors. Eur J Pharmacol 307: 331–338.
Gelfand EV, Cannon CP (2006). Rimonabant: a selective blocker of the cannabinoid CB1 receptors for the management of obesity, smoking cessation and cardiometabolic risk factors. Expert Opin Investig Drugs 15: 307–315.
Gifford AN, Makriyannis A, Volkow ND, Gatley SJ (2002). In vivo imaging of the brain cannabinoid receptor. Chem Phys Lipids 121: 65–72.
Hawkins RA, Phelps ME, Huang S-C (1986). Effects of temporal sampling, glucose metabolic rates, and disruptions of the blood-brain barrier on the FDG model with and without a vascular compartment: studies in human brain tumors with PET. J Cereb Blood Flow Metab 6: 170–183.
Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR et al (1990). Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA 87: 1932–1936.
Hirst RA, Almond SL, Lambert DG (1996). Characterisation of the rat cerebella CB1 receptor using SR141716A, a central cannabinoid receptor antagonist. Neurosci Lett 220: 101–104.
Horti AG, Fan H, Kuwabara H, Hilton J, Ravert HT, Holt DP et al (2006). 11C-JHU75528: a radiotracer for PET Imaging of CB1 cannabinoid receptors. J Nucl Med 47: 1689–1696.
Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA et al (2002). International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54: 161–202.
Katoch-Rouse R, Pavlova OA, Caulder T, Hoffman AF, Mukhin AG, Horti AG (2003). Synthesis, structure-activity relationship, and evaluation of SR141716 analogues: development of central cannabinoid receptor ligands with lower lipophilicity. J Med Chem 46: 642–645.
Khawaja X, Evans N, Reilly Y, Ennis C, Minchin MC (1995). Characterisation of the binding of [3H]WAY-100635, a novel 5-hydroxytryptamine1A receptor antagonist, to rat brain. J Neurochem 64: 2716–2726.
Kirkham TC, Tucci SA (2006). Endocannabinoids in appetite control and the treatment of obesity. CNS Neurol Disord Drug Targets 5: 272–292.
Laruelle M, Baldwin RM, Rattner Z, Al-Tikriti MS, Zea-Ponce Y, Zoghbi SS et al (1994). SPECT quantification of [123I]iomazenil binding to benzodiazepine receptors in nonhuman primates. I. Kinetic modeling of single bolus experiments. J Cereb Blood Flow Metab 14: 439–452.
Le Foll B, Goldberg SR (2005). Cannabinoid CB1 receptor antagonists as promising new medications for drug dependence. J Pharmacol Exp Ther 312: 875–883.
Mathews WB, Scheffel U, Finley P, Ravert HT, Frank RA, Rinaldi-Carmona M et al (2000). Biodistribution of [18F] SR144385 and [18F] SR147963: selective radioligands for positron emission tomographic studies of brain cannabinoid receptors. Nucl Med Biol 27: 757–762.
Mathews WB, Scheffel U, Rauseo PA, Ravert HT, Frank RA, Ellames GJ et al (2002). Carbon-11 labeled radioligands for imaging brain cannabinoid receptors. Nucl Med Biol 29: 671–677.
Nyberg S, Chou YH, Halldin C (2002). Saturation of striatal D2 dopamine receptors by clozapine. Int J Neuropsychopharmacol 5: 11–16.
Pfister JR, Makra F, Muehldorf AV, Wu H, Nelson JT, Cheung P et al (1995). Methanodibenzosuberylpiperazines as potent multidrug resistance reversal agents. Bioorg Med Chem Lett 5: 2473–2476.
Roe M, Folkes A, Ashworth P, Brumwell J, Chima L, Hunjan S et al (1999). Reversal of P-glycoprotein mediated multidrug resistance by novel anthranilamide derivatives. Bioorg Med Chem Lett 9: 595–600.
Sadeque AJ, Wandel C, He H, Shah S, Wood AJ (2000). Increased drug delivery to the brain by P-glycoprotein inhibition. Clin Pharmacol Ther 68: 231–237.
Schinkel AH, Wagenaar E, Mol CA, van Deemter L (1996). P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest 97: 2517–2524.
Simoneau II, Hamza MS, Mata HP, Siegel EM, Vanderah TW, Porreca F et al (2001). The cannabinoid agonist WIN55,212-2 suppresses opioid-induced emesis in ferrets. Anesthesiology 94: 882–887.
Tamagan G, Lu X-J, Gao Y, Amici LA, Baldwin RM, Innis RB (1999). Potential SPECT radioligand for cannabinoid receptor. J Label Compd Radiopharm 42: S191–S193.
Walker JM, Hohmann AG, Martin WJ, Strangman NM, Huang SM, Tsou K (1999). The neurobiology of cannabinoid analgesia. Life Sci 65: 665–673.
Yasuno F, Hasnine AH, Suhara T, Ichimiya T, Sudo Y, Inoue M et al (2002). Template-based method for multiple volumes of interest of human brain PET images. Neuroimage 16: 577–586.
Yasuno F, Zoghbi SS, McCarron JA, Ichise M, Brown AK, Gladding RL et al (2006). Quantification of serotonin 5-HT1A receptors in monkey brain with [11C](R)-()-RWAY. Synapse 60: 510–520.
Zoghbi SS, Baldwin RM, Seibyl JS, Charney DS, Innis RB (1993). A radiometric technique for determining apparent pKa of receptor-binding ligands. J Label Compd Radiopharm 40S: 136–138.
Zoghbi SS, Shetty HU, Ichise M, Fujita M, Imaizumi M, Liow J-S et al (2005). PET imaging of the dopamine transporter with [18F]FECNT: a polar radiolabeled metabolite confounds brain radioligand measurements. J Nucl Med 47: 520–527.
Acknowledgements
We thank Jonathan P Gourley, BS for help with radiometabolite analysis; Jeih-San Liow for assistance on image analysis; and the staff of the NIH PET Department for successful completion of the scanning studies, and Xenova Group, Ltd (UK) for providing tariquidar. This research was performed via a Cooperative Research and Development Agreement (CRADA) with Eli Lilly, Karolinska Institutet, and NIMH and was supported in part by the Intramural Program of NIMH (project Z01-MH-002795-04). FY was funded by Japanese Society for the Promotion of Sciences, Research Fellow in Biomedical and Behavioral Research at NIH (2005–2006). Eli Lilly provided research grant support to NIMH and the Karolinska Institutet as part of the current CRADA or other research agreements. RBI and VWP (NIMH) have received research support, but no personal income, from Eli Lilly and AstraZeneca. CH (Karolinska Institutet) has received research support and/or personal income from Eli Lilly and AstraZeneca.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yasuno, F., Brown, A., Zoghbi, S. et al. The PET Radioligand [11C]MePPEP Binds Reversibly and with High Specific Signal to Cannabinoid CB1 Receptors in Nonhuman Primate Brain. Neuropsychopharmacol 33, 259–269 (2008). https://doi.org/10.1038/sj.npp.1301402
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301402
Keywords
This article is cited by
-
Association between cannabinoid 1 receptor availability and glutamate levels in healthy controls and drug-free patients with first episode psychosis: a multi-modal PET and 1H-MRS study
European Archives of Psychiatry and Clinical Neuroscience (2021)
-
Classification of advanced methods for evaluating neurotoxicity
Molecular & Cellular Toxicology (2021)
-
Expression and localization of CB1R, NAPE-PLD, and FAAH in the vervet monkey nucleus accumbens
Scientific Reports (2018)
-
Acute induction of anxiety in humans by delta-9-tetrahydrocannabinol related to amygdalar cannabinoid-1 (CB1) receptors
Scientific Reports (2017)
-
Synthesis and Preclinical Evaluation of [18F]FCHC for Neuroimaging of Fatty Acid Amide Hydrolase
Molecular Imaging and Biology (2015)