Abstract
To advance understanding of the neurochemical changes in Parkinson's disease (PD), we compared D2-like dopamine receptor occupancy by dopamine in the control and lesioned putamen of four pig-tailed macaques treated unilaterally with MPTP. PET and in vitro binding techniques were used to measure binding potential (BP*) and density of D2-like dopamine receptors (Bmax), respectively. As would be expected in PD, relatively higher values of BP* and Bmax and less amphetamine-induced decrease in [11C]raclopride binding were observed in the lesioned compared with the contralateral putamen in each animal. The percent differences between lesioned and contralateral sides were similar whether the measurements were of [11C]raclopride BP* or Bmax values, measured in vivo and in vitro, respectively. As [11C]raclopride BP* is a measure of the density of D2-like dopamine receptors available for radioligand binding (i.e., not occupied by dopamine), these findings suggest that the fractional occupancy of receptors by endogenous dopamine in the lesioned putamen is nearly equal to that in the contralateral putamen. Therefore, the absolute number of receptors occupied by dopamine, which is a product of receptor density and fractional occupancy by dopamine, is greater in the lesioned than in the contralateral putamen. One possible explanation for the lack of differences in fractional occupancy of D2 receptors by dopamine (despite a loss in available dopamine) is a lesion-induced increase in a portion of low-affinity D2 receptors to a state of high affinity for dopamine.
Similar content being viewed by others
INTRODUCTION
The hallmark of Parkinson's disease (PD) is an extensive loss of dopaminergic neurons in the substantia nigra, leading to degeneration of the nigrostriatal dopaminergic projection and loss of striatal dopamine (Bernheimer et al, 1973; Kish et al, 1988). In the non-human primate MPTP model of PD, a microdialysis study showed that the level of extracellular dopamine in the striatum ipsilateral to MPTP injection was less than one-tenth of that measured on the contralateral side (Skirboll et al, 1990; Gerhardt et al, 1999). In addition, PET studies have demonstrated that amphetamine-induced decrease in specific binding of [11C]raclopride, a marker for dopamine release, was below control values in PD patients and MPTP-treated animals (Doudet and Holden, 2003; Piccini et al, 2003). Previous studies indicated that both D2-like dopamine receptor density measured in vivo (Bmaxin vivo) and binding potential of [11C]raclopride (BP*), the latter of which is an in vivo measure of a density of receptors available for radioligand binding, are elevated in the brains of untreated patients with PD and in the MPTP animal model compared with control values.
D2-like receptors in striatum exist in at least three compartments: an extracellular membrane-bound high-affinity population, an extracellular membrane-bound low-affinity population, and an intracellular population. [11C]raclopride binds to D2-like receptors in all compartments (Man Jiang et al, 2006). Therefore, both Bmax and BP* measurements reflect the sum of D2-like receptors in all three compartments. Whereas Bmax provides a measure of total D2-like receptor density, BP* characterizes the density of D2-like receptors not bound to dopamine and available for raclopride binding. Consequently, the ratio of BP* to Bmax (BP*/Bmax) provides a measure of receptor occupancy by dopamine for entire population of striatal D2-like receptors.
Because [11C]raclopride binding in vivo is sensitive to extracellular dopamine levels and BP* values increase after dopamine depletion without changes in Bmaxin vivo (Ginovart et al, 1997), it is reasonable to expect that in PD, the difference from control would be greater when measuring BP* than Bmaxin vivo. However, published results show that the differences between control and PD cases were nearly equal whether measurements were of BP* or of Bmaxin vivo (Doudet et al, 2002; Doudet and Holden, 2003; Rinne et al, 1995). These observations suggest that the fraction of receptors available for binding dopamine, and consequently, the fractional occupancy of D2 receptors by dopamine, is not changed in PD. Several considerations should be taken into account, however, to accept this conclusion.
First, Bmax estimations in vivo can be influenced by several methodological issues. For example, the existence of D2-like receptors in more than one affinity state for dopamine may complicate estimation of the portion of these receptors in a high-affinity state. In addition, administration of D2 receptor antagonists, including raclopride, at high doses during saturation studies may facilitate dopamine release (Andersson et al, 1995; Ishizu et al, 2000), leading to an underestimation of Bmax. This underestimation would be expected to be less pronounced in patients with PD than in healthy control subjects.
Second, the progression of PD may result in differences between PD patients and healthy control subjects in parameters of specific and nonspecific binding of [11C]raclopride. Such alterations might include a decrease in the true receptor affinity (1/KD) for [11C]raclopride, as has been observed with [3H]spiroperidol binding in postmortem brain tissue samples from some Parkinsonian patients (Rinne et al, 1981) and MPTP-treated monkeys (Alexander et al, 1991). In addition, changes of tissue composition in the brains of PD patients may affect nonspecific binding of the radiotracer, thereby affecting the measurement of non-displaceable volume of distribution (VDnd) and, therefore, of BP*.
Finally, even though [11C]raclopride binding to D2-like receptors, assessed by PET, is widely used to monitor changes in synaptic dopamine concentration, the sensitivity of this method is relatively low. Data obtained with PET and microdialysis on the same animal show that a doubling of extracellular dopamine in the striatum reduces [11C]raclopride binding by only 1–1.5% (Breier et al, 1999; Tsukada et al, 1999).
The purpose of this study was to verify that the fractional occupancy of D2 dopamine receptors is unchanged in the MPTP model of PD despite presynaptic dopamine loss. To overcome the issues and concerns discussed above, we used a unilateral MPTP model of PD in non-human primates to measure BP*, VDnd, and Bmax in vivo and Bmax and KD in vitro, bilaterally in the putamen of the same animal. Comparing data obtained under the same experimental condition in the right and left striatum of the same MPTP-lesioned animals minimized the error associated with PET measurements.
MATERIALS AND METHODS
All experiments involving research animals were approved by the Animal Care and Use Committee (ACUC) of the NIDA Intramural Research Program.
Four hemiparkinsonian, pig-tailed macaques (Macaca nemestriana), 5–10 kg, had received a single unilateral intracarotid injection of MPTP 10 to 12 years before the PET studies (Emborg-Knott and Domino, 1998). Asymmetry in motor behavior remained stable in these monkeys up to date when the PET studies were performed (Domino et al, 2003). The lesioned monkeys exhibited severe symptoms, including hypokinesia, rigidity, bradykinesia, and circling behavior contralateral to the MPTP injections (Domino et al, 2003). Loss of presynaptic dopaminergic terminals in the lesioned striatum was documented by a loss of vesicular monoamine transporter (Kilbourn et al, 2001) and in nicotinic acetylcholine receptors (Chefer et al, 2002), which are presumably located on presynaptic dopamine terminals.
In Vivo PET Studies
Animals were prepared for PET and MRI scans as described previously (Chefer et al, 2003). Briefly, each monkey was initially anesthetized with 1.5 mg/kg alfadolone and alfaxolone acetate (Saffan®; Arnolds Veterinary Products, Shropshire, UK) administered intramuscularly. Anesthesia was maintained throughout the scanning by a continuous intravenous (i.v.) infusion of 9–14 mg/kg/h Saffan. For PET studies, an individually molded thermoplastic face mask allowing reproducible positioning between studies was secured to a monkey head-holder attached to a backboard. Data were acquired on a Siemens Exact ECAT HR+ whole-body tomograph in 3-D mode (PET procedures and image reconstruction were described previously; Chefer et al, 2003). For MRI, the monkey's head was placed in an MRI-compatible stereotaxic frame. MRI scans were acquired with a General Electric 1.5 T Signa MR unit (GE Medical Systems, Milwaukee, WI, USA) at the NIH MRI Research Facility in Bethesda (MD, USA) following procedures described previously (Matochik et al, 2000, 2004). Vital signs, including heart rate, EKG, respiration rate, and oxygen saturation, were continuously monitored during the studies. Blood oxygen saturation was always maintained above 90%.
Regions of interest (ROIs) were drawn on magnetic resonance images co-registered to the PET images. Briefly, the MRIs were registered to the corresponding PET images by using a normalized mutual information algorithm. First, the skull and extracranial tissue on each monkey's structural MRI were removed using the image edit auto-trace function in ANALYZE©. A standard ROI template was constructed using the MEDx 3.42 image analysis program (Sensor Systems, Sterling, VA). Each ROI was a rectangle enclosing 6 × 8 pixels and an area of 15.6 mm2. The ROIs were constructed bilaterally on four consecutive slices to sample the caudate nucleus and putamen and on one slice to sample the cerebellum. The standard ROI template, manually adjusted for individual differences in brain structure for each monkey MRI image, was transferred to the corresponding PET scans. No adjustments of ROIs were performed after placement on the PET scans. Thus, ROI placement was guided solely by the neuroanatomy of each brain rather than the distribution of the radioligand. Time–activity curves for the cerebellum and left and right caudate nucleus and putamen were constructed.
In all PET studies, [11C]raclopride was administered intravenously as a bolus followed by a constant infusion (B/I) to reach an equilibrium in radioactivity distribution. The bolus component delivered a mass of radiotracer equivalent to that administered in the 210 min of infusion that followed (Kbolus=210 min). Acquisition of dynamic PET scans started with the beginning of the bolus component of the radioligand administration and continued for 140 min (30 frames of increasing duration).
Two series of PET studies with [11C]raclopride were performed on each of three animals. In the first series of experiments, BP* and Bmaxin vivo were measured. In the second, the effect of d-amphetamine challenge was tested. A fourth animal was involved in the first series of studies for BP* measurement only, and was excluded from the second series of PET experiments for technical reasons. To estimate Bmaxin vivo values in caudate nucleus and putamen, raclopride was administered to each animal in three doses (ca. 0.5, 13, and 80 nmol/kg) in one PET study comprised of three sessions (one dose per session) starting with lowest mass dose. These doses were prepared by administering approximately 0.8 mCi/kg of [11C]raclopride with specific activities of approximately 1500, 60, and 10 Ci/mmol, respectively, over 2 h as B/I. The specific activity of [11C]raclopride was varied by adding known amounts of unlabeled raclopride to radioactive compound. The interval between the start of each PET session was 2.5 h.
Bmaxin vivo in the caudate nucleus and the putamen were calculated using Scatchard analysis. After a near-equilibrium condition was reached (ca. 60 min after the beginning of ligand administration, the level of radioactivity in the brain structures remained unchanged to the end of the scanning session), the specifically bound radioactivity in caudate nucleus and putamen was calculated as the difference between the total radioactivity measured in the caudate nucleus or putamen and that in the cerebellum (Cb). As a region devoid of D2-like dopamine receptors, the cerebellum provides an estimate of the sum of free and nonspecifically bound radioligand. BP*, which is defined as the ratio between the volume of distribution of specific binding compartment and the volume of distribution of non-displaceable compartment (Ichise et al, 2001), was calculated as (ROI-Cb)/Cb. (ROI-Cb) was plotted against (ROI-Cb)/Cb. Only data obtained from PET studies with the highest [11C]raclopride specific activity were used to calculate BP*.
In the second series of experiments, each of three monkeys participated in a PET study, with two sessions performed on the same day. In the first session, monkeys received saline and in the second session, they received amphetamine. The interval between the beginning of the sessions was 160–170 min. In each session, the animal received 1.3–1.6 mCi/kg of [11C]raclopride (SA=3000–4000 Ci/mmol) administered over 140 min as B/I with Kbolus=210 min. Amphetamine (0.4 mg/kg, i.v.) or saline was injected 60 min after the beginning of B/I [11C]raclopride administration. Amphetamine-induced decrease in [11C]raclopride binding was calculated for each animal as the difference between BP* values observed at 60 min after saline injection and BP* values after amphetamine injection (120 min after the start of [11C]raclopride administration). The difference was expressed as the percentage of the BP* value in the control (saline) study. Radioactivity 120 min after the beginning of [11C]raclopride administration was taken as an average of radioactivity from 100 to 140 min. Two additional PET studies using unlabelled raclopride as a displacing agent were performed on two animals to compare the VDnd in contralateral and lesioned striatum. Studies of unlabelled raclopride-induced displacement produced data similar to those obtained with d-amphetamine. Unlabelled raclopride (1 mg/kg, i.v.) was injected as a single bolus over 20 s at 50 min after the beginning of B/I [11C]raclopride administration. Non-displaceable radioactivity was assessed 60 min later (110 min after the beginning of [11C]raclopride administration). Radioactivity at 110 min after the beginning of [11C]raclopride administration was actually an average of radioactivity from 100 to 120 min.
In Vitro Binding Assays
After the completion of the last PET study and on the same day, the monkeys were euthanized by an overdose of pentobarbital. The brain was rapidly removed from the skull and the whole putamen from each hemisphere was dissected separately and frozen at −70°C. Frozen tissue samples were homogenized in HEPES–salt solution (1:20 (w:v), tissue to solution) and kept at −70°C in 1 ml aliquots containing 50–70 mg wet tissue. HEPES–salt solution, pH 7.4, contains 15 mM HEPES, 120 mM NaCl, 5.4 mM KCl, 0.8 mM MgCl2, and 1.8 mM CaCl2. On the day of assay, the homogenate was rehomogenized in incubation buffer (50 mM Tris–HCl, 120 mM NaCl, 5 mM KCl, 2 mM CaCl2, and 1 mM MgCl2; 1:250, w:v, tissue to solution) and centrifuged at 50000g for 20 min. Saturation assays with [3H]raclopride were performed as described previously (Kohler et al, 1985). Briefly, pellets were rehomogenized in the incubation buffer. In triplicate assays, aliquots of homogenate, 0.05 ml (corresponding to 1.8 mg wet tissue) were incubated for 1.5 h at 23°C with seven concentrations of [3H]raclopride (0.3–22 nM) in a final volume of 0.075 ml. Nonspecific binding was determined in the presence of 10 μM raclopride.
Statistical Analysis
Statistical analysis was performed using SigmaStat (version 2.0) software. It had been shown that both Bmax and BP* are elevated in untreated patients with PD and in an animal model of PD compared with control values (Rinne et al, 1995; Doudet et al, 2002; Doudet and Holden, 2003). Therefore, to confirm that the animals in this study had an increased Bmax and BP* for raclopride in the lesioned putamen compared with the contralateral side, as is typical for PD, we used one-tailed paired t-tests. The null hypothesis was that BP* and D2 receptor density was decreased or unchanged on the lesioned side compared with the contralateral side. Differences between KD values on the lesioned and contralateral sides in vitro were tested by two-tailed paired t-tests. Student's t-test was applied only after the data passed tests of normality and equality of variance. Pearson Product Moment Correlation and Spearman Rank Order Correlation analyses were used to estimate the relationship between Bmax values measured in vivo and in vitro and the percent difference between contralateral and lesioned putamen in Bmaxin vitro and BP*.
Theory
BP*, defined as the ratio of the distribution volume for the specific binding compartment to that for the nondisplaceable compartment (VDsb/VDnd) (Ichise et al, 2001), is proportional to the density of binding sites available for radioligand (Bmax−B) and radioligand affinity (1/KD) (Carson et al, 1997; Laruelle et al, 1994). BP* can be expressed as

where (Bmax–B) is the number of D2-like dopamine receptors available for radioligand binding, KD is the dissociation constant of radioligand–receptor complexes, S is a fraction of receptors occupied by dopamine, and VDnd is the nondisplaceable volume of distribution.
Therefore, in MPTP-lesioned animals, BP* of raclopride in contralateral and lesioned striatum (BP*C and BP*L, respectively) can be expressed as


Assuming that the VDnd and KD of [11C]raclopride are not different comparing the lesioned and contralateral sides (VDnd(C)=VDnd(L) and KD(C)=KD(L), respectively), the following equation can be derived by dividing the left- and right-hand terms of equation (2) by the corresponding terms of equation (3):

where SL and SC are the fractions of D2-like receptors occupied by dopamine on the lesioned and contralateral sides. From equation (4), we can find if  the fraction of D2-like receptors occupied by dopamine on the lesioned side is equal to that on the contralateral side.
the fraction of D2-like receptors occupied by dopamine on the lesioned side is equal to that on the contralateral side.
RESULTS
The lesioned putamen showed significantly higher values of BP* and Bmaxin vivo compared with those on the contralateral side in all of the animals tested (Figures 1 and 2). The BP* and Bmaxin vivo values in the lesioned putamen were 37% (P<0.02) and 34% (P<0.03) greater, respectively (Figure 3). Relative differences in the caudate nucleus were less pronounced (14 and 19% higher for BP* and Bmaxin vivo on the lesioned side, respectively) and not statistically significant (Figure 3).
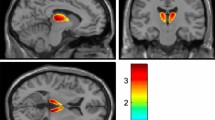
Representative BP* parametric brain images for [11C]raclopride coregistered with the MR image of the same animal. Animal was treated with MPTP through the right carotid artery. Top row, MR image; middle row, BP* image; and bottom row, BP* images fused with MR image. (a) Transaxial view, (b) coronal view, and (c) saggital view (left and right). BP* images were obtained by pixel-wise modeling using a simplified reference region method (Gunn et al, 1997).
Scatchard plots of the data obtained for the putamen in in vivo saturation studies with [11C]raclopride. For each animal, the data were obtained from three PET scans performed on the same day using radioligand with different specific activities (see details in Materials and methods). Open and filled symbols represent the data for the putamen on the ipsilateral and contralateral to the MPTP injection sides, respectively.
Binding potential (BP*), D2-like dopamine receptor density (Bmax), and affinity (KDapp) on the lesioned (open columns) and contralateral (filled columns) sides in hemiparkinsonian pig-tailed macaques measured in vivo. Data represent mean±SEM (n=4 for BP* and n=3 for Bmax and KDapp). The values on the lesioned side were expressed as % of that on the contralateral side (100%).
Amphetamine administration induced dopamine release in the caudate nucleus and the putamen on both sides, reducing [11C]raclopride binding (Figure 4). The BP* values were reduced by 23% in the putamen and by 19% in the caudate nucleus on the side contralateral to the lesion after amphetamine administration (Figure 5). Amphetamine challenge was less effective in releasing dopamine on the lesioned side where the decreases in BP* in the putamen and caudate nuclei were 6 and 12%, respectively. The amphetamine-induced decrease in BP* of [11C]raclopride in the putamen on the lesioned side was one-fourth that measured on the contralateral side (Figure 5). The difference between values in the caudate nucleus of the lesioned and contralataeral sides was smaller than in the putamen (1.5-fold). Thus, of the four sites tested, the smallest response to amphetamine challenge was observed in the lesioned putamen, which also showed the highest D2-like dopamine receptor density and probably reflected a lesion of greater severity.
Effect of d-amphetamine administration on kinetics of [11C]raclopride accumulation in putamen on contralateral and lesioned sides. Data represent typical time–activity curves in putamen (circles) and cerebellum (triangles) after B/I administration of [11C]raclopride. Data were obtained from two PET scans performed on the same day on one animal challenged with saline (filled symbols) or amphetamine (open symbols) at 60 min after the beginning of radioligand administration as shown by arrow.
D-amphetamine-induced decrease in binding potential (BP*) in striatum on the lesioned (open columns) and contralateral (filled columns) sides. Data represent mean percent change (n=3) in BP* values±SEM after amphetamine challenge (0.4 mg/kg, i.v) compared with saline injection (P<0.02 using one-tailed paired t-test).
Displacement studies with raclopride were performed in monkeys 1 and 3. Treatment with unlabelled raclopride (1 mg/kg, i.v.) 50 min after starting the bolus/infusion of [11C]raclopride resulted in a rapid washout of radioactivity from the putamen (T1/2=13±1 min) to the level of radioactivity in the cerebellum. The ratios of nondisplaceable radioactivity in lesioned putamen to that in contralateral putamen, assessed 60 min after unlabelled raclopride injection (110 min after the beginning of [11C]raclopride administration), were 0.99 and 1.05 for monkey 1 and 3, respectively. These results suggest that VDnd for [11C]raclopride does not differ significantly when comparing the contralateral and lesioned putamen.
In vitro binding assays were carried out on eight samples of brain tissue, four samples from the lesioned putamen, and four samples from the contralateral putamen. Since dopamine level was expected to differ in the lesioned and contralateral sides, the assays were performed in washed homogenates and in the presence of 120 mM NaCl to minimize the effects of endogenous dopamine on radioligand binding. Scatchard plots of the data from in vitro binding assays performed under these conditions were linear (Figure 6), suggesting that [3H]raclopride interacted with a single population of binding sites in vitro. The KD values for [3H]raclopride measured in vitro (Table 1) were nearly identical in lesioned and contralateral putamen (2.1±0.1 and 2.0±0.2 nM, respectively). Bmaxin vitro values were 35% higher in the lesioned putamen compared with those measured in contralateral putamen (Bmaxin vitro=16±1 and 22±2 pmol/g tissue in contralateral and lesioned putamen, respectively; P<0.01) (Table 1). There was high correlation between Bmaxin vitro and Bmaxin vivo values (r=0.89, P<0.01) (Figure 7), but in vitro estimations were consistently lower (by 22 and 24% in contralateral and lesioned putamen, respectively) compared with their respective Bmaxin vivo values (Table 1). Lower Bmaxin vitro values compared with Bmaxin vivo probably reflected incomplete recovery of the receptors after homogenization and washing procedures. The percent difference between contralateral and lesioned putamen in Bmaxin vitro correlated strongly with the percent difference in BP* (r=0.92, P<0.05). Consistent with this observation,  (see Theory section, equation 4) were nearly equal to 1 in all four animals. Taken together, these data suggest that the increase in BP* values on the lesioned side is solely a result of a greater receptor density.
(see Theory section, equation 4) were nearly equal to 1 in all four animals. Taken together, these data suggest that the increase in BP* values on the lesioned side is solely a result of a greater receptor density.
DISCUSSION
The experiments described here, on hemiparkinsonian monkeys, demonstrated that the percent increase in [11C]raclopride BP* values in the lesioned compared with contralateral putamen was nearly equal to the elevations of Bmax measured in vivo and in vitro. Since BP* is a measure of the density of D2-like receptors available for radioligand binding (ie not occupied by dopamine), this observation suggests that the fractional occupancy of receptors by endogenous dopamine in the lesioned putamen is unchanged.
The animals used in our study exhibited alterations in dopaminergic markers on the lesioned side typical of untreated PD patients and of the MPTP model of PD in animals. These included an increase in BP*, elevation in Bmax, and reduction in amphetamine-induced decrease of [11C]raclopride specific binding (Rinne et al, 1995; Perez-Otano et al, 1994; Doudet et al, 2000). In addition, the findings were more pronounced in the putamen than in the caudate nucleus. This pattern is also common for PD and is typical of the MPTP model of PD in non-human primates (Rinne et al, 1995; Doudet and Holden, 2003).
The monkeys were anesthetized in our study. Although isoflurane has a profound effect on raclopride binding both at baseline and after methamphetamine injection (Tsukada et al, 2002), we are not aware of any data showing the effect of Saffan®, which we used, on [11C]raclopride binding and its interaction with amphetamine. Nevertheless, since the comparison was made between the lesioned and non-lesioned side under the same experimental condition, we believe that any effect of anesthesia has little importance in interpretation of the results of current study.
Notably, values above control BP* (Rinne et al, 1993, 1995; Antonini et al, 1994, 1997; Wenning et al, 1998; Ichise et al, 1999; Kaasinen et al, 2000) and D2 receptor density (Rinne et al, 1995) were revealed by PET and SPECT studies in untreated patients at early stages of PD. The absence of changes or a decrease in BP* in patients with advanced PD (Antonini et al, 1997; Linazasoro et al, 1999; Thobois et al, 2004) may result, in part, from long-term administration of therapeutic doses of antiparkinsonian medications such as L-DOPA and/or dopamine agonists (Thobois et al, 2004). Supporting this view are observations of a decrease in the number of D2 sites below control levels in L-DOPA-treated parkinsonian monkeys (Alexander et al, 1991) and an upregulation of D2 receptors in putamen in late-stage PD after discontinuation of dopaminergic drug therapy (Thobois et al, 2004). It is possible that L-DOPA treatment converts D2 receptors from a high-affinity state to a low-affinity state for dopamine, as has been suggested (Borbely et al, 1999).
Similar KD and VDnd values in the lesioned compared with contralateral putamen suggest that the difference between lesioned and contralateral putamen in BP* depends strictly on the density of D2 receptor available for radioligand binding (BP*=Bmaxav/KD/VDnd). With a deficit in dopamine on the lesioned side, a greater relative increase in BP* values compared with the total receptor density (Bmax) was expected. However, we observed that the relative increase in BP* in the lesioned putamen was very similar to that in Bmax measured in vitro, suggesting near identical fractional receptor occupancy by dopamine on both sides. Since the total receptor density is elevated in the lesioned putamen and fractional occupancy is unchanged, we can conclude that the absolute number of receptors occupied by dopamine in the lesioned putamen is greater than in the contralateral putamen.
Despite potential methodological problems associated with using PET and [11C]raclopride for Bmax measurements (see introduction), the differences in Bmax in the lesioned (vs contralateral) putamen measured in vitro and in vivo were nearly equal. In addition, there was a strong correlation between Bmax values measured in vitro and in vivo (r=0.89). Taken together, these data validate the utilization of Bmax values from in vivo studies to estimate receptor occupancy by dopamine the same way as was utilized for Bmax in vitro.
In addition to the experimental determinations, differences in BP* and Bmax between lesioned and contralateral putamen were calculated using published data from studies where BP* and Bmaxin vivo only were measured in PD patients (Rinne et al, 1995) and in MPTP-treated non-human primates (Doudet et al, 2002; Doudet and Holden, 2003). As shown in Table 2, the results from four independent studies, including the present one, are in good agreement. These data reveal that the percentage of changes in BP* is nearly equal or even smaller than that in Bmax and suggest that the fractional receptor occupancy by dopamine in the lesioned putamen is not lower, as might be expected because of a dopamine deficit. This implies that the absolute number of D2 receptors occupied by dopamine in PD is greater compared with normal control values.
Our findings suggest that upregulation of D2-like dopamine receptor in PD is not a direct compensation for a decrease in the fractional occupancy of these sites by dopamine due to a DA deficit, as ruled out previously (Rinne et al, 1995; Doudet et al, 2002). Still it is possible that increased receptor density reflects a homeostatic mechanism to compensate for the deficit of receptor occupancy by dopamine during active neurotransmission (ie during phasic dopamine release), rather than the baseline condition (tonic dopamine release). This upregulation might result in overcompensation of receptor occupancy by dopamine during low level of dopaminergic transmission.
Theoretically, the absence of changes in fractional occupancy of D2-like receptors in lesioned putamen could be explained either by a lack of loss in the level of dopamine on the lesioned side or by the elevated affinity of D2-like receptors for dopamine. Data of Robinson and Whishaw (1988), showing that an extracellular concentration of dopamine measured by microdialysis was normal on the lesioned side in unanaesthetized rats treated unilaterally with 6-OHDA, support the idea that the dopamine concentration is unchanged in PD. However, at least three studies in MPTP-treated non-human primates using microdialysis showed a robust decline in dopamine on the lesioned side (Skirboll et al, 1990; Gerhardt et al, 1999; Oiwa et al, 2003), favoring the idea of receptor affinity changes in PD. An increased affinity of D2 receptors for dopamine could mask an effect of neurotransmitter loss on binding.
One of the compensatory mechanisms for the loss of dopaminergic input in PD may be a shift of a portion of low-affinity D2-like receptors to a high-affinity state. Such an effect could also contribute to the development of dopamine hypersensitivity in PD (Lee et al, 1978). In this regard, experimental manipulations that compromise the dopaminergic system in animals produced an increase in the fraction of D2-like receptors in a high-affinity state for dopamine (Seeman et al, 2005). A second mechanism leading to an increase in D2 receptor affinity for dopamine could be a translocation of these receptors from an intracellular pool, where they are not accessible to dopamine, to the membrane surface. Further studies using new radioligands with agonistic properties at D2-like dopamine receptors (Narendran et al, 2005; Seneca et al, 2006; Willeit et al, 2006) could advance the testing of these hypotheses and be useful to directly examine the fraction of D2-like dopamine receptors in a high affinity state in PD and its animal models.
References
Alexander GM, Brainard DL, Gordon SW, Hichens M, Grothusen JR, Schwartzman RJ (1991). Dopamine receptor changes in untreated and (+)-PHNO-treated MPTP parkinsonian primates. Brain Res 547: 181–189.
Andersson JL, Nomikos GG, Marcus M, Hertel P, Mathe JM, Svensson TH (1995). Ritanserin potentiates the stimulatory effects of raclopride on neuronal activity and dopamine release selectivity in the mesolimbic dopaminergic system. Naunyn Schmiedebergs Arch Pharmacol 352: 374–385.
Antonini A, Schwarz J, Oertel WH, Beer HF, Madeja UD, Leenders KL (1994). [11C]raclopride and positron emission tomography in previously untreated patients with Parkinson's disease: Influence of L-dopa and lisuride therapy on striatal dopamine D2-receptors. Neurology 44: 1325–1329.
Antonini A, Schwarz J, Oertel WH, Pogarell O, Leenders KL (1997). Long-term changes of striatal dopamine D2 receptors in patients with Parkinson's disease: a study with positron emission tomography and [11C]raclopride. Mov Disord 12: 33–38.
Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F (1973). Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 20: 415–455.
Borbely K, Brooks RA, Wong DF, Burns RS, Cumming P, Gjedde A et al (1999). NMSP binding to dopamine and serotonin receptors in MPTP-induced parkinsonism: relation to dopa therapy. Acta Neurol Scand 100: 42–52.
Breier A, Su TP, Malhotra AK, Elman I, Adler CM, Weisenfeld NI et al (1999). Effects of atypical antipsychotic drug treatment on amphetamine-induced striatal dopamine release in patients with psychotic disorders. Neuropsychopharmacology 20: 340–345.
Carson RE, Breier A, de Bartolomeis A, Saunders RC, Su TP, Schmall B et al (1997). Quantification of amphetamine-induced changes in [11C]raclopride binding with continuous infusion. J Cereb Blood Flow Metab 17: 437–447.
Chefer SI, London ED, Koren AO, Pavlova OA, Kurian V, Kimes AS et al (2003). Graphical analysis of 2-FA binding to nicotinic acetylcholine receptors in Rhesus monkey brain. Synapse 48: 25–34.
Chefer SI, Mukhin AG, Koren AO, Kimes AS, Pavlova OA, Matochik JA et al (2002). Striatal α4β2 nicotinic acetylcholine receptors in the MPTP model of parkinson's desease: PET study with 2-[18F]-fluoro-A-85380. NeuroImage 16: S21.
Domino EF, Ni L, Colpaert F, Marien M (2003). Effects of (+/−)-idazoxan alone and in combination with L-DOPA methyl ester in MPTP-induced hemiparkinsonian monkeys. Receptors Channels 9: 335–338.
Doudet DJ, Holden JE (2003). Raclopride studies of dopamine release: dependence on presynaptic integrity. J Cereb Blood Flow Metab 12: 1489–1494.
Doudet DJ, Holden JE, Jivan S, McGeer E, Wyatt RJ (2000). In vivo PET studies of the dopamine D2 receptors in rhesus monkeys with long-term MPTP-induced parkinsonism. Synapse 38: 105–113.
Doudet DJ, Jivan S, Ruth TJ, Holden JE (2002). Density and affinity of the dopamine D2 receptors in aged symptomatic and asymptomatic MPTP-treated monkeys: PET studies with [11C]raclopride. Synapse 44: 198–202.
Emborg-Knott M, Domino E (1998). MPTP-Induced hemiparkinsonism in nonhuman primates 6-8 years after a single unilateral intracarotid dose. Exp Neurol 152: 214–220.
Gerhardt GA, Cass WA, Huettl P, Brock S, Zhang Z, Gash DM (1999). GDNF improves dopamine function in the substantia nigra but not the putamen of unilateral MPTP-lesioned rhesus monkeys. Brain Res 817: 163–171.
Ginovart N, Farde L, Halldin C, Swahn CG (1997). Effect of reserpine-induced depletion of synaptic dopamine on [11C]raclopride binding to D2-dopamine receptors in the monkey brain. Synapse 25: 321–325.
Gunn RN, Lammertsma AA, Hume SP, Cunningham VJ (1997). Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage 6: 279–287.
Ichise M, Kim YJ, Ballinger JR, Vines D, Erami SS, Tanaka F et al (1999). SPECT imaging of pre- and postsynaptic dopaminergic alterations in L-dopa-untreated PD. Neurology 52: 1206–1214.
Ichise M, Meyer JH, Yonekura Y (2001). An introduction to PET and SPECT neuroreceptor quantification models. J Nucl Med 42: 755–763.
Ishizu K, Smith DF, Bender D, Danielsen E, Hansen SB, Wong DF et al (2000). Positron emission tomography of radioligand binding in porcine striatum in vivo: haloperidol inhibition linked to endogenous ligand release. Synapse 38: 87–101.
Kaasinen V, Nagren K, Hietala J, Oikonen V, Vilkman H, Farde L et al (2000). Extrastriatal dopamine D2 and D3 receptors in early and advanced Parkinson's disease. Neurology 54: 1482–1487.
Kilbourn KR, Frey KS, Bankiewicz LN, Domino EF (2001). Persistent MPTP induced hemiparkinsonian lesions in monkeys 9-11 years later: [11C]Dihydrotetrabanazine ([11C]DTZ) as an in vivo PET ligand. Soc Neurosci 26: 1028 (abstract).
Kish SJ, Shannak K, Hornykiewicz O (1988). Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson's disease. Pathophysiologic and clinical implications. N Engl J Med 318: 876–880.
Kohler C, Hall H, Ogren SO, Gawell L (1985). Specific in vitro and in vivo binding of 3H-raclopride. A potent substituted benzamide drug with high affinity for dopamine D-2 receptors in the rat brain. Biochem Pharm 34: 2251–2259.
Laruelle M, van Dyck C, Abi-Dargham A, Zea-Ponce Y, Zoghbi SS, Charney DS et al (1994). Compartmental modeling of iodine-123-iodobenzofuran binding to dopamine D2 receptors in healthy subjects. J Nucl Med 35: 743–754.
Lee T, Seeman P, Rajput A, Farley IJ, Hornykiewicz O (1978). Receptor basis for dopaminergic supersensitivity in Parkinson's disease. Nature 273: 59–61.
Linazasoro G, Obeso JA, Gomez JC, Martinez M, Antonini A, Leenders KL (1999). Modification of dopamine D2 receptor activity by pergolide in Parkinson's disease: an in vivo study by PET. Clin Neuropharmacol 22: 277–280.
Man Jiang, Guo W, Scheiren I, Narendran R, Javitch J, Rayport S et al (2006). Agonistmediated internalization of dopamine D2 receptors does not appear to mediate the decrease in benzamides binding potential observed after dopamine surge. NeuroImage 31: T32.
Matochik JA, Chefer SI, Lane MA, Roth GS, Mattison JA, London ED et al (2004). Age-related decline in striatal volume in monkeys: assessment of long-term calorie restriction. Neurobiol Aging 25: 5193–5200.
Matochik JA, Chefer SI, Lane MA, Woolf RI, Morris ED, Ingram DN et al (2000). Age-related decline in striatal volume in monkeys as measured by magnetic resonance imaging. Neurobiol Aging 21: 591–598.
Narendran R, Hwang DR, Slifstein M, Hwang Y, Huang Y, Ekelund J et al (2005). Measurement of the proportion of D2 receptors configured in state of high affinity for agonists in vivo: a positron emission tomography study using [11C]N-propyl-norapomorphine and [11C]raclopride in baboons. J Pharmacol Exp Ther 315: 80–90.
Oiwa Y, Eberling JL, Nagy D, Pivirotto P, Emborg ME, Bankiewicz KS (2003). Overlesioned hemiparkinsonian non-human primate model: correlation between clinical, neurochemical and histochemical changes. Front Biosci 8: 155–166.
Perez-Otano I, Oset C, Luquin MR, Herrero MT, Obeso JA, Del Rio J (1994). MPTP-induced parkinsonism in primates: pattern of striatal dopamine loss following acute and chronic administration. Neurosci Lett 175: 121–125.
Piccini P, Pavese N, Brooks DJ (2003). Endogenous dopamine release after pharmacological challenges in Parkinson's disease. Ann Neurol 53: 647–653.
Rinne JO, Laihinen A, Rinne UK, Nagren K, Bergman J, Ruotsalainen U (1993). PET study on striatal dopamine D2 receptor changes during the progression of early Parkinson's disease. Mov Disord 2: 134–138.
Rinne JO, Laihinen A, Ruottinen H, Ruotsalainen U, Nagren K, Lehikoinen P et al (1995). Increased density of dopamine D2 receptors in the putamen, but not in the caudate nucleus nucleus in early Parkinson's disease: a PET study with [11C]raclopride. J Neurol Sci 132: 156–161.
Rinne UK, Lonnberg P, Koskinen V (1981). Dopamine receptors in the Parkinsonian brain. J Neural Transm 51: 97–106.
Robinson TE, Whishaw IQ (1988). Normalization of extracellular dopamine in striatum following recovery from a partial unilateral 6-OHDA lesion of the substantia nigra: a microdialysis study in freely moving rats. Brain Res 450: 209–224.
Seeman P, Weinshenker D, Quirion R, Srivastava LK, Bhardwaj SK, Grandy DK et al (2005). Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc Natl Acad Sci USA 102: 3513–3518.
Seneca N, Finnema SJ, Farde L, Gulyas B, Wikstrom HV, Halldin C et al (2006). Effect of amphetamine on dopamine D2 receptor binding in nonhuman primate brain: a comparison of the agonist radioligand [11C]MNPA and antagonist [11C]raclopride. Synapse 59: 260–269.
Skirboll S, Wang J, Mefford I, Hsiao J, Bankiewicz KS (1990). In vivo changes of catecholamines in hemiparkinsonian monkeys measured by microdialysis. Exp Neurol 110: 187–193.
Thobois S, Vingerhoets F, Fraix V, Xie-Brustolin J, Mollion H, Costes N et al (2004). Role of dopaminergic treatment in dopamine receptor down-regulation in advanced Parkinson disease: a positron emission tomographic study. Arch Neurol 61: 1705–1709.
Tsukada H, Miyasato K, Kakiuchi T, Nishiyama S, Harada N, Domino EF (2002). Comparative effects of methamphetamine and nicotine on the striatal [(11)C]raclopride binding in unanesthetized monkeys. Synapse 45: 207–212.
Tsukada H, Nishiyama S, Kakiuchi T, Ohba H, Sato K, Harada N (1999). Is synaptic dopamine concentration the exclusive factor which alters the in vivo binding of [11C]raclopride? PET studies combined with microdialysis in conscious monkeys. Brain Res 841: 160–169.
Wenning GK, Donnemiller E, Granata R, Riccabona G, Poewe W (1998). 123I-beta-CIT and 123I-IBZM-SPECT scanning in levodopa-naive Parkinson's disease. Mov Disord 13: 435–443.
Willeit M, Ginovart N, Kapur S, Houle S, Hussey D, Seeman P et al (2006). High-affinity states of human brain dopamine D2/3 receptors imaged by the agonist [11C]-(+)-PHNO. Biol Psychiatry 59: 389–394.
Acknowledgements
This study was supported by the Intramural Research Program of the National Institute on Drug Abuse, NIH, DHHS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chefer, S., Kimes, A., Matochik, J. et al. Estimation of D2-like Receptor Occupancy by Dopamine in the Putamen of Hemiparkinsonian Monkeys. Neuropsychopharmacol 33, 270–278 (2008). https://doi.org/10.1038/sj.npp.1301404
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1301404
Keywords
This article is cited by
-
Translational molecular imaging and drug development in Parkinson’s disease
Molecular Neurodegeneration (2023)
-
Exercise Improves Cognitive Impairment and Dopamine Metabolism in MPTP-Treated Mice
Neurotoxicity Research (2016)