Abstract
Central serotonin function was studied among 21 adolescents (12 males, 9 females), mean age 14.4±1.5 years. A placebo-controlled design was used to measure three neuroendocrine hormones (prolactin, cortisol and growth hormone) following a challenge with the central serotonergic agonist m-chlorophenylpiperazine (mCPP). Infusion of mCPP resulted in augmented prolactin, cortisol and growth hormone release. Gender effects were significant for prolactin, cortisol and growth hormone. Females had higher baseline prolactin without significant interactions with infusion or time, cortisol levels were higher in males than in females at all time points without significant interactions with infusion or time, and the augmented growth hormone response to mCPP was limited to males. Systolic and diastolic blood pressure, heart rate and temperature were all mildly elevated following mCPP infusion. Side effects to mCPP infusion were mild and lasted approximately 20 min. We conclude that mCPP is useful in the study of serotonergic neuroendocrine hormones in adolescents, is well tolerated, and the levels of prolactin, cortisol and growth hormone are influenced by gender.
Similar content being viewed by others
INTRODUCTION
m-Chlorophenylpiperazine (mCPP) is a research tool to study central serotonin function. It is a metabolite of the antidepressants trazodone and nefazodone, and its administration leads to hormonal, physiological and behavioral effects. It is a potent serotonin postsynaptic receptor agonist with action at 5-HT1, 5-HT1A, 5-HT2 and 5-HT2C and with antagonistic action at 5-HT3 (Hamik and Peroutka, 1989; Kahn and Wetzler, 1991). Of these, its binding affinity is probably most potent at the 5-HT1C receptors (Hoyer, 1988). These effects have been studied among adults with psychiatric disorders and normal controls (Kahn et al, 1988,1990b; Anand et al, 1994). An augmented neuroendocrine hormone response is generally considered indicative of receptor hypersensitivity and a blunted response of hyposensitivity (Murphy and Mueller, 1986; Van Praag et al, 1987). When 5-HT receptor antagonists such as methysergide and metergoline are administered prior to mCPP challenge, suppression of hormones, (prolactin, cortisol), body temperature, and behavioral responses to oral mCPP follow (Kahn et al, 1990a; Kalus et al, 1990). These studies have provided evidence that mCPP acts via central 5-HT receptors to release cortisol, prolactin, and possibly growth hormone (GH) and corticotropin (ACTH), and that it influences temperature and behavior. Recently, presynaptic functions of mCPP have also been noted because of its binding with serotonin transporter (Baumann et al, 1995). Overall, mCPP as a serotonergic probe has been well studied among adults, and such studies have resulted in useful data regarding the pathophysiology of adult major depression.
There are limited data regarding the use of mCPP among adolescents. The only publication we know of involved a group of depressed adolescents and normal controls (Ghaziuddin et al, 2000). Key findings of this study were that the depressed group had augmented prolactin and cortisol responses to an infusion of mCPP, compared with the normal controls (Ghaziuddin et al, 2000). Similar to adults, therefore, mCPP has potential as a useful research tool for studying serotonergic pathophysiology among adolescents with depression.
mCPP has several advantages as a pharmacological research tool for the study of the central serotonergic system. These include a dose-dependent increase in prolactin, cortisol and temperature, action on central postsynaptic serotonin receptors, a short half-life, ease of administration, and a relative lack of side effects (Mueller et al, 1985a,1985b). Few data have been collected however.
The present study was undertaken to further identify the effects of mCPP among adolescents, and to determine if serotonin-related neuroendocrine hormones (prolactin, GH and cortisol) are influenced by gender. We hypothesized that gender is a significant factor in the neuroendocrine hormone responses of boys and girls challenged with the pharmacological agent mCPP.
METHODS
Subjects
Participants were recruited from newspaper advertisements and flyers posted throughout a university hospital. Subjects had never received any psychiatric diagnosis on Axis I or on Axis II, and did not have first-degree relatives with a psychiatric disorder. Other exclusions to participation were pregnancy, lactation, drug abuse or dependence, major medical disorder, and mental retardation or pervasive developmental disorder. All participants received a comprehensive psychiatric evaluation (a clinical interview with a child psychiatrist and a structured, computerized diagnostic interview using the Diagnostic Interview Schedule for Children (DISC); Costello et al, 1985; Fisher et al, 1993), a physical examination, and routine laboratory tests (complete blood cell count, liver function tests, thyroid functions, urine toxicology, serum pregnancy for females and EKG). A child psychiatrist using information from the clinical and structured diagnostic interviews determined the final psychiatric diagnoses. Subjects completed a battery of psychometric instruments: the Children's Depression Rating Scale—Revised (CDRS-R; Poznanski et al, 1985), a clinician-rated scale; the Hamilton Depression Rating Scale (HDRS; Hamilton, 1960), a clinician-rated scale; the Severity of Suicidal Behavior (SSB; Pfeffer, 1986), a clinician-rated scale; the Child Behavior Checklist (CBCL; Achenbach, 1991a), completed by an adult who knows the child; the Youth Self Report (YSR; Achenbach, 1991b), completed by the adolescent; and the Children's Global Assessment Scale (CGAS; Endicott and Spitzer, 1976), based on assessment made by a clinician. Each subject also completed the Behavior Checklist (NIMH; Van Kammen and Murphy, 1975) at multiple time points to address emotional and physical symptoms experienced before and during the challenge test.
Participants also completed Tanner staging on a standard form by selecting pictures of appropriate secondary sexual characteristics. All female participants were studied during the first 14 days of their menstrual cycle. Socioeconomic status was determined using the Stevens and Featherman (1981) scale. All participants were asked to adhere to a low-monoamine diet for approximately 48 h prior to the study.
Written informed consent from a parent or legal guardian and assent of the subject were obtained. The Institutional Review Board (IRB) approved the study protocol. Approval for the use of mCPP was obtained from the US Food and Drug Administration; IND 46,227.
mCPP Challenge
Participants were admitted the evening prior to the study to the General Clinical Research Center (GCRC), a specialized facility designed for pharmacological, physiologic, and metabolic studies, where they underwent testing for serotonergic function.
Subjects were asked not to sleep or eat during the study period. Drinking water was permitted. Physical activity was limited from midnight prior to the study, and subjects were permitted to get up only for bathroom purposes. Each subject received saline and mCPP challenges, using a fixed-order (saline followed by mCPP), single-blind design (the investigators, but not the subject or staff members, were aware of the order). The fixed-order design was used because it was not feasible to hospitalize adolescents for more than one night and so to conduct the challenge over two days. Also, the challenge order could not be counterbalanced (eg mCPP first, followed by saline for some participants), because clearance of mCPP from the body occurs over several hours.
At 0700 h, an intravenous catheter was placed in the forearm vein for serial blood sampling. After a 50-min period of stabilization following catheter placement, subjects received a slow intravenous push of 20 ml saline administered over 90 s. Subjects completed the Behavior Checklist just prior to drug or placebo administration and at 3, 10, 20, 35, 50, and 90 min after infusion. Blood samples were collected at 10 and 3 min before infusion and at 20, 35, 50, 95, and 120 min after saline infusion. Recording of vital signs (blood pressure (BP), heart rate and temperature) was carried out just prior to infusion, and at each blood draw. Subjects received 0.1 mg/kg mCPP in saline, infused over 90 s under conditions identical to the saline infusion, 1 h after the last blood draw for the saline challenge. The Behavior Checklist, monitoring of vital signs and serial blood sampling were completed as for the saline challenge. Blood was collected into Vacutainer tubes (Becton Dickinson) containing ethylene-diaminetetraacetic acid (EDTA), placed on ice, and centrifuged immediately after collection. Plasma was aliquoted and stored at −70°C until assayed. Subjects remained in the hospital bed for an additional 30 min after completion of the protocol. They were served lunch and discharged.
Hormone Assays
Prolactin
Prolactin (PRL) was measured in plasma samples by a standard radioimmunoassay procedure (Stuart et al, 1982) using rabbit prolactin antiserum, sheep anti-rabbit gamma-globulin and 125I-labeled prolactin. The assay sensitivity is 0.14 ng/ml, the interassay coefficient of variation is 6.9%, and the intra-assay coefficient of variation is 3.5%.
Cortisol
Cortisol was measured in ethanol extracts of plasma samples by a standard competitive protein-binding method (De La Pena and Goldzieher, 1974) using human cortisol-binding globulin. The assay sensitivity is 0.2 μg/dl, interassay coefficient of variation is 5.2%, and the intra-assay coefficient of variation is 4.0%.
Growth Hormone
Growth hormone was assayed with a double-antibody assay using 125I-labeled GH as a tracer. The first antibody is incubated with the specimens for 1 h. After incubation, the tracer is added and incubation takes place over another hour. Subsequently, the precipitating antibody is added. Supernatant is decanted, and the precipitate is counted with a gamma counter. The concentration of GH is calculated from a standard curve. The detection limit was approximately 0.9 ng/ml; the interassay coefficient of variation is 4.4% and the intra-assay coefficient of variation is 3.0%.
Analyses
Males and females were compared on a range of continuous variables using independent sample t-tests: SES, CDRS, HRSD, YSR (total, internalizing and externalizing subscales) and CBCL (total, internalizing and externalizing). Fisher's exact test was used for the comparisons of categorical variables, such as Tanner stage, season of testing and SSB. Baseline hormone levels were calculated as the mean of values at −10 and −3 min and were compared using a two-way, repeated-measures ANOVA, with gender as a between-subject factor and time (pre-saline or pre-mCPP) as a within-subject factor. Both PRL and cortisol were analyzed on the natural log scale to make the response more normally distributed; GH was analyzed on the original scale. The comparisons of hormone levels and vital signs (temperature, systolic BP diastolic BP, and heart rate) for males and females over time were performed using a repeated-measures ANOVA, with gender as a between-subject effect, and time and infusion (saline or mCPP) as within-subject effects.
Side effects (headache, stomachache, dizziness, sweating, tremors, blurred vision, nausea, and tremor) that were significantly greater during mCPP infusion compared with saline infusion were used as covariates in the comparison of vital signs. Repeated-measures ANOVA was also used to analyze NIMH symptoms, which were based on average symptom score (the average of all symptom scores reported at a given time point) and number of severe symptoms (the number of symptoms with a score of 5 or greater on a scale from 0 to 10).
RESULTS
There were 21 adolescents (9 females and 12 males, mean age=14.6±1.5 years). The females and males were similar in age, Tanner rating and SES. There was, however, a significant difference between males and females in the season of testing (Fisher's exact test, p=0.015): the majority of the females (n=7, 77.7%) underwent the challenge procedure in the fall; for males, the most frequent time was the summer (n=6, 54.5%). Males and females were similar in all behavior ratings.
Prolactin
Baseline PRL levels (Table 1) at 0800 h (prior to saline infusion) and at 1100 h (prior to mCPP infusion) were compared using a two-way, repeated-measures ANOVA. There was a significant gender effect (F=6.6, df=1, 19, p=0.019), with PRL higher in females than in males; a significant time effect (F=25.3, df=1, 19, p=0.0001), with both males and females experiencing a decline in PRL from 0800 to 1100 h; and no significant interaction between gender and time.
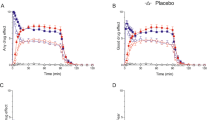
Next, a repeated-measures analysis of covariance (ANCOVA) was computed to compare the effect of saline and mCPP infusions over time for males and females, adjusting for baseline PRL levels and for the presence of nausea (nausea can influence neuroendocrine hormone levels). We did not find a significant gender effect (F=1.3, df=1, 19, p=0.263), nor were any interactions with gender significant, although females had consistently higher PRL levels than males at each time point for both infusions (Figure 1). The time effect was not significant, nor was there a significant time by infusion interaction, although there was a trend for both of these effects. A highly significant effect of infusion was found (F=301.88, df=1, 19, p⩽0.0001), with PRL levels generally being higher for mCPP infusion than for saline. The presence of nausea (nausea score > 0) was a significant predictor of higher log PRL levels (F=5.08, df=1, 30, p=0.025). The maximum PRL level following mCPP infusion was reached slightly earlier in males (peak at 30 min) than in females (peak at 50 min). After the maximum was reached, a decline was observed in both genders; this decline was more rapid for males than for females. The PRL levels did not return to pre-mCPP values during the 2 h after mCPP infusion.
Cortisol
Baseline cortisol levels (Table 1) at 0800 h (prior to saline infusion) and 1100 h (prior to mCPP infusion) were compared using a two-way, repeated-measures ANOVA. There was a significant effect of time (F=9.2, df=1, 18, p=0.007), with a higher cortisol level noted for both genders at 0800 h than at 1100 h.
Next, a repeated-measures ANCOVA was computed to compare the effect of saline and mCPP infusions over time for males and females, adjusting for baseline cortisol levels and nausea. There was no gender effect and no significant gender by time interaction. Nausea had no effect on cortisol levels. There was a significant gender effect (F=5.47, df=1, 18, p=0.031), with males having generally higher cortisol levels than females. None of the interactions with gender was significant. In both genders, the peak cortisol level was reached at 40 min post-mCPP, followed by a return to baseline by 120 min (Figure 2). A significant time effect (F=25.4, df=4, 7, p⩽0.0001) was found. A significant effect of infusion was also noted (F=194.68, df=1, 18, p⩽0.0001), with a higher cortisol level following mCPP than saline. There was a signifi-cant time by infusion interaction (F=8.30, df=4, 72, p⩽0.0001), with cortisol levels remaining generally constant or slightly declining over time after saline infusion, but rising sharply and then declining after mCPP infusion.
Growth Hormone
Baseline GH levels (Table 1) at 0800 h (prior to saline infusion) and at 1100 h (prior to mCPP infusion) were compared using a two-way, repeated-measures ANOVA. There was no significant gender effect, time effect, or gender by time interaction. Unlike PRL and cortisol, GH levels did not change significantly between 0800 and 1100 h.
Next, a repeated-measures ANCOVA was performed to identify the effect of gender, time and infusion, adjusting for baseline GH levels. The time effect was not significant, although GH levels increased after infusion of both saline and mCPP and then declined (Figure 3). There was no overall effect of gender, but there was a significant gender by infusion interaction (F=10.43, df=1, 17, p=0.004). Males showed a significantly higher response to mCPP than to saline (F=23.31, df=1, 17, p=0.0002); females showed no significant difference between mCPP and saline infusion.
Side Effects
Altogether 22 symptoms from the NIMH symptom list were assessed for each participant at seven time points (3 min before infusion and at 3, 10, 20, 35, 50, and 95 min after) for both saline and mCPP infusions. Each symptom was rated on a scale from 0 to 10, where 0=‘not very much’ and 10=‘very much’. A symptom was counted as present if the score was greater than zero. The average symptom score for each patient was calculated at each time point. The number of severe symptoms (score ≥5) was also calculated at each time point, because it was believed that this level was likely to cause distress in most participants.
A repeated-measures ANOVA was performed for the average number of symptoms experienced at each time point, with gender as a between-subjects effect and infusion and time as within-subjects effects. Post hoc tests were performed using the Tukey–Kramer adjustment for multiple comparisons. The effect of gender was not significant, but infusion effect (F=9.95, df=1, 19, p=0.0052) and time effect (F=10.23, df=6, 114, p=0.0001) were significant. Also, a significant time by infusion interaction (F=10.97, df=6, 114, p=0.0001) was found. As a result of the highly significant interaction between time and treatment, we tested the effect of time separately for each treatment. We found no significant time effect for the saline treatment (F=0.19, df=6, 114, p=0.98), but there was a highly significant time effect for the mCPP treatment (F=21.01, df=6, 114, p=0.0001). Both infusions started out with very similar average symptom scores at baseline. For the mCPP infusion, however, the number of symptoms increased sharply at 3 min postinfusion, remained elevated for approximately the first 20 min, and then began a sharp decline. The average symptom scores, although higher after mCPP infusion, were still not very high. Post hoc tests on infusion revealed no significant difference at baseline (t=0.67, df=114, p>0.99) but significant differences at 3 min (t=4.86, df=114, p=0.0003), 10 min (t=4.68, df=114, p=0.0006) and 20 min (t=4.49, df=114, p=0.0014). There were no significant differences between the two infusions for any subsequent time points. Average symptom scores for females were consistently higher than for males following mCPP infusion, but there were no significant main gender effects or interactions in the model. In summary, the average number of side effects experienced by males and females during the first 20 min after the mCPP infusion was significantly greater than after the saline infusion.
Next, males and females were compared on the number of severe symptoms (score ≥5) experienced after saline or mCPP infusion. There was a significant time effect (F=2.2, df=6, 114, p=0.05), but no gender or infusion effect. In other words, there was no significant difference in severe symptoms following saline vs mCPP infusions.
Vital Signs
A repeated-measures ANOVA of systolic BP revealed an infusion effect, with higher systolic pressure following mCPP infusion (F=27.1, df=1, 19, p<0.0001), a significant time effect (F=2.6, df=6, 114, p=0.022), and a significant effect of headache (F=5.1, df=1, 244, p=0.025). Those with headache had a higher systolic BP; however, there was no effect of gender and no interaction between these variables. For diastolic BP, a significant effect of infusion was noted, with higher diastolic pressure following mCPP infusion (F=14, df=1, 19, p=0.001). There was a significant gender effect (F=4.5, df=1, 19, p=0.047), with females having a higher diastolic BP than males, and a significant effect of stomachache (F=5.9, df=1, 245, p=0.016). Higher diastolic BP was associated with stomachache; however, the interaction between these variables was not significant. For heart rate, although the main effect of infusion was not significant, the interaction between time and infusion was significant (F=2.4, df=6, 113, p=0.033), with higher heart rate noted only at 3 min post-mCPP. The interaction between gender and infusion was also significant (F=4.7, df=1, 19, p=0.05), with females having a higher heart rate at 3 min post-mCPP infusion. Sweating also had a significant effect (F=5.07, df=1, 245, p=0.025) and was associated with higher heart rate. For temperature, there was a significant effect of infusion (F=13.5, df=1, 19, p=0.001), with a higher temperature following mCPP than saline, and a significant time effect (F=2.5, df=7, 129, p=0.017), but there was no effect of gender or any of the side effects.
In summary, blood pressure, temperature and heart rate were elevated after mCPP. Only heart rate and diastolic BP were influenced by gender. Some of the side effects influenced BP and heart rate, but temperature was not influenced by any of the side effects.
DISCUSSION
The main findings of the present study are:
-
1
Females had a higher baseline PRL level compared with males. Although infusion with mCPP resulted in significantly greater response, gender did not influence the response to mCPP. Nausea was a significant predictor for PRL release. There was diurnal variation in PRL for both males and females, with significantly higher levels at 0800 h (saline baseline) than at 1100 h (mCPP baseline). Gender, time of day, presence of nausea, and mCPP all influenced the level of PRL.
-
2
Diurnal variation was noted for cortisol for both boys and girls, with a significantly higher level at 0800 h than at 1100 h. Cortisol level was significantly higher after mCPP infusion, compared with saline infusion. Although the level for boys and girls was similar at baseline, over the course of the mCPP infusion, cortisol levels were higher for males than for females; however, the difference did not reach significance. Nausea had no effect on cortisol levels.
-
3
Boys and girls had similar GH levels at baseline. The diurnal variation noted for PRL and cortisol was absent for GH and the levels at 0800 and 1100 h were similar. Only boys had a higher GH response to mCPP; girls did not display a similarly augmented response. There was a significant time effect after both saline and mCPP infusion.
-
4
There were no significant differences in the side effects experienced by boys and girls. Nausea increased PRL response but did not affect cortisol. Both boys and girls experienced peak symptom intensity during the first 20 min, with a decline to baseline levels by 50 min. The average symptom scores, although greater after mCPP infusion, were still not exceptionally high.
-
5
Systolic BP, diastolic BP, heart rate and temperature were all mildly elevated following mCPP infusion. Side effects such as headache, stomachache and sweating influenced BP and heart rate but not temperature. There were no gender differences for systolic BP or temperature; however, females had a higher diastolic BP than males, and only females had a higher heart rate in response to mCPP.
The augmented PRL, cortisol, and GH responses noted in the present study are strongly consistent with findings in healthy adults, which suggests that normal control adolescents and healthy adults overall respond similarly to intravenous mCPP challenge (Charney et al, 1987; Lawlor et al, 1989a,1989b). We cannot compare gender differences in adolescents with adults, however, as there are no adult reports involving male–female comparisons. Further, it is noteworthy that similar neuroendocrine responsivity noted among healthy adolescents and healthy adults may not generalize to depressed patients. For instance, as part of an ongoing larger study, we have earlier reported (Ghaziuddin et al, 2000) augmented PRL and cortisol responses to mCPP among depressed adolescents, which was in contrast to reduced PRL responsivity found in the majority of studies involving depressed adults (Maes et al, 1989; Cowen and Charig, 1987; Lopez-Ibor et al, 1989). It is possible that dissimilar neuroendocrine responses among depressed adolescents and adults, despite similar neuroendocrine responses among healthy adolescents and healthy adults, may reflect an interaction between developmental and depressed status. Noteworthy gender differences evident for PRL, cortisol and GH among the adolescents suggest that gender must be controlled for in the measurement of these neuroendocrine hormones.
Age- and gender-related differences in the PRL levels are supported by animal data. In the rat, for instance, Neill (1972) reported fundamental gender-based differences in the secretion of PRL, which were established shortly after birth and were neural in origin. Similarly, Yamamoto et al (1970) found a rapid age-related PRL synthesis and release in both male and female newborn rats; however, the increase was greater and more rapid among females. The same study also found a greater GH release in male than in female rats; the synthesis of GH was little affected by age in females. In adult human males and females, however, another study involving the measurement of PRL secretion over a 24-h period did not find a gender difference (Sassin et al, 1972).
Examination of heart rate, BP, and temperature revealed that these physiologic measures were only mildly elevated after mCPP infusion and unlikely to be deleterious to health. The mechanisms underlying these observations, however, are not entirely clear. Two possibilities are that these are either serotonergic effects of mCPP, or are stress-related responses resulting from side effects such as stomachache, headache or sweating. Cardiovascular effects of 5-HT are known to vary between species, within species, and within the same individual at successive testing (Goodman and Gilman, 1970). A study involving experimental rats failed to find a significant effect of mCPP infusion on mean arterial pressure or heart rate (Cohen et al, 1987). Similarly, most studies involving adult humans have found either no effect on heart rate (Kahn et al, 1990b) or no significant changes in both BP and heart rate (Silverstone et al, 1994; Seibyl et al, 1991). The most likely explanations for raised BP and heart rate among adolescents are either that these observations are stress-related responses or that they represent atypical development-related response to mCPP. Increased temperature, on the other hand, was unrelated to side effects and was possibly a direct effect of mCPP. Overall, side effects were mild and brief, lasting approximately 50 min, after which these observations returned to baseline levels. We concluded that mCPP was well tolerated by adole-scents and that the side effects were of moderate severity, short-lived, and causing minimum discomfort to patients.
CONCLUSION
To our knowledge, this is the first published study of serotonergic neuroendocrine hormones in normal control adolescents who are at a low risk for developing a psychiatric disorder (because of a negative family psychiatric history). Neuroendocrine hormones may be affected by several factors, including age, gender, puberty, presence of psychiatric illness, baseline vs challenged responses, choice of pharmacological challenge agent, and route of administration of the challenge agent. This study underscores the role of gender using the present methodology.
Limitations of this study include a relatively small sample size, a single-blind design for the infusion, and lack of gender-related adult data for meaningful comparisons. Also, the effect of age or sexual maturation could not be examined because the majority of the patients had reached Tanner stage 5, which is consistent with the complete development of secondary sexual characteristics, and the subjects therefore did not represent early puberty.
References
Achenbach TM (1991a). Integrative Guide for the 1991 CBCL/4-18, YSR and TRF Profiles. Department of Psychiatry, University of Vermont: Burlington.
Achenbach TM (1991b). Manual for the Youth Self Report and 1991 Profile. Department of Psychiatry, University of Vermont: Burlington.
Anand A, Charney DS, Delgado PL, McDougle CJ, Heninger GR, Price LH (1994). Neuroendocrine and behavioral responses to intravenous m-chlorophenylpiperazine (mCPP) in depressed patients and healthy comparison subjects. Am J Psychiatry 151: 1626–1630.
Baumann MH, Mash DC, Staley JK (1995). The serotonin agonist m-chlorophenylpiperazine (mCPP) binds to serotonin transporter sites in human brain. Neuroreport 6: 2150–2152.
Charney DS, Woods SW, Goodman WK, Heninger GR (1987). Serotonin function in anxiety: II. Effects of serotonin agonist mCPP in panic disorder patients and healthy subjects. Psychopharmacology 92: 14–24.
Cohen ML, Kurz KD, Fuller RW (1987). Effects of meta-chlorophenylpiperazine (mCPP), a central serotonin agonist and vascular serotonin receptor antagonist, on blood pressure in SHR. Clin Exp Hypertens A 9: 1549–1565.
Costello EJ, Edelbrock CS, Costello AJ (1985). Validity of the NIMH diagnostic interview schedule for children: a comparison between psychiatric and pediatric referrals. J Abnorm Child Psychol 47: 579–595.
Cowen PJ, Charig EM (1987). Neuroendocrine responses to intravenous tryptophan in major depression. Arch Gen Psychiatry 44: 958–966.
De La Pena A, Goldzieher JW (1974). Practical determination of total plasma cortisol by use of competitive protein binding. Clin Chem 20: 1376–1378.
Endicott J, Spitzer RL (1976). The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry 33: 766–771.
Fisher PV, Shaffer D, Placentini JD, Lapkin J, Kafantaris V, Leonard HC (1993). Sensitivity of the diagnostic interview schedule for children, 2nd edn DISC-2: I. For specific diagnoses of children and adolescents. J Am Acad Child Adolesc Psychiatry 32: 666–673.
Ghaziuddin N, King CA, Welch KB, Zaccagnini J, Weidmer-Mikhail E, Mellow A (2000). Preliminary report: serotonin dysregulation in adolescents with major depression: hormone response to meta-chlorophenylpiperazine (mCPP) infusion. Psychiatry Res 95: 183–184.
Goodman LS, Gilman A (1970). The Pharmacological Basis of Therapeutics. Macmillan: New York.
Hamilton M (1960). A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62.
Hamik A, Peroutka SJ (1989). 1-(m-Chlorophenyl)piperazine (mCPP) interactions with neurotransmitter receptors in the human brain. Biol Psychiatry 25: 569–575.
Hoyer D (1988). Functional correlates of serotonin 5-HT1 recognition sites. J Recept Res 8: 59–81.
Kahn RS, Asnis GM, Wetzler S, van Praag HM (1988). Neuroendocrine evidence for serotonin receptor hypersensitivity in panic disorder. Psychopharmacology (Berl) 96: 360–364.
Kahn RS, Kalus O, Wetzler S, Cahn W, Asnis GM, van Praag HM (1990a). Effects of serotonin antagonists on m-chlorophenylpiperazine-mediated responses in normal subjects. Psychiatry Res 33: 189–198.
Kahn RS, Wetzler S, Asnis GM, Papolos D, van Praag HM (1990b). Serotonin receptor sensitivity in major depression. Biol Psychiatry 28: 358–362.
Kahn RS, Wetzler S (1991). m-Chlorophenylpiperazine as a probe of serotonin function. Biol Psychiatry 30: 1139–1166.
Kalus O, Kahn RS, Wetzler S, Asnis GM, van Praag HM (1990). Hypersensitivity to m-chlorophenylpiperazine in a subject with subclinical panic attacks. Biol Psychiatry 28: 1053–1057.
Lawlor BA, Sunderland T, Hill JL (1989a). Evidence for a decline with age in behavioral responsivity to the serotonin agonist m-chlorophenylpiperazine, in healthy human subjects. Psychiatry Res 29: 1–10.
Lawlor BA, Sunderland T, Mellow AM, Hill JL, Molchan SE, Murphy DL (1989b). Hyper-responsivity to the serotonin agonist, m-chlorophenylpiperazine in Alzheimer's disease. Arch Gen Psychiatry 46: 542–549.
Lopez Jr JJ, Saiz-Ruiz J, Moral Iglesias L (1989). Neuroendocrine challenges in the diagnosis of depressive disorders. Br J Psychiatry 154: 73–76.
Maes M, Vandewoude M, Schotte C, Maes L, Martin M (1989). Sex-linked differences in cortisol, ACTH and prolactin responses to 5-hydroxytryptophan in healthy controls and minor and major depressed patients. Acta Psychiatr Scand 80: 584–590.
Mueller EA, Murphy DL, Sunderland T, Jones J (1985a). A new postsynaptic serotonin receptor agonist suitable for studies in humans. Psychopharmacol Bull 21: 1179–1184.
Mueller EA, Sunderland T, Murphy DL (1985b). Neuroendocrine effects of mCPP, a serotonin agonist, in humans. J Clin Endocrinol Metab 61: 1179–1184.
Murphy DL, Mueller EA (1986). Use of serotonergic agents in the clinical assessment of central serotonin function. J Clin Psychiatry 47: 9–15.
Neill JD (1972). Sexual differences in the hypothalamic regulation of prolactin secretion. Endocrinology 90: 1154–1159.
Pfeffer CR (1986). The Suicidal Child. Guilford Press: New York.
Poznanski E, Mokros HB, Grossman J, Freeman LN (1985). Diagnostic criteria in childhood depression. Am J Psychiatry 142(10): 1168–1173.
Sassin JF, Weitzman ED, Kapen S (1972). Human prolactin 24-hour pattern with increased release during sleep. Science 177: 1205–1207.
Seibyl JP, Krystal JH, Price LH, Woods SW, D'Amico C, Heninger GR (1991). Effects of ritanserin on the behavioral, neuro-endocrine, and cardiovascular responses to m-chlorophenyl-piperazine in healthy human subjects. Psychiatry Res 38: 227–236.
Silverstone PH, Rue JE, Hallis FK, Camplin G, Lave D, Cowen PJ (1994). The effects of administration of mCPP on psychological, cognitive, cardiovascular, hormonal and MHPG measurements in human volunteers. Int Clin Psychopharmacol 9: 173–178.
Stevens G, Featherman DL (1981). Occupational status index. Soc Sci Res 10: 364–395.
Stuart MC, Underwood PA, Boscato L (1982). A monoclonal antibody suitable for the radioimmunoassay of prolactin in human serum. J Clin Endocrinol Metab 54: 881–884.
Van Praag HM, Lemus L, Kahn R (1987). Hormonal probes of central serotonergic activity: do they really exist? Biol Psychiatry 22: 86–98.
Van Praag HM, Lemus L, Kahn R (1987). Hormonal probes of central serotonergic activity: do they really exist? Biol Psychiatry 22: 86–98.
Van Kammen DP, Murphy DL (1975). Attenuation of the euphoriant and activating effects of D- and L-amphetamine by lithium carbonate treatment. Psychopharmacologia 44: 215–224.
Yamamoto Y, Taylor LM, Cole F (1970). Synthesis and release of GH and prolactin from the rat anterior pituitary in vitro as functions of age and sex. Endocrinology 87: 21–26.
Acknowledgements
This study was supported by the National Alliance for Research on Schizophrenia and Depression (NARSAD) and the US National Institutes of Health (NIH) (M01-RR00042).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghaziuddin, N., Welch, K. & Greden, J. Central Serotonergic Effects of m-Chlorophenylpiperazine (mCPP) among Normal Control Adolescents. Neuropsychopharmacol 28, 133–139 (2003). https://doi.org/10.1038/sj.npp.1300006
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300006
Keywords
This article is cited by
-
Nieuwe Psychoactieve Stoffen (NPS): niets nieuws onder de zon
Verslaving (2013)
-
Pharmacology of ayahuasca administered in two repeated doses
Psychopharmacology (2012)
-
Pharmacological management of appetite expression in obesity
Nature Reviews Endocrinology (2010)
-
Comparison of the Effects of Citalopram and Escitalopram on 5-Ht-Mediated Neuroendocrine Responses
Neuropsychopharmacology (2004)