Abstract
Cocaine tolerance was assessed by comparing the acute effects of cocaine in drug-abstinent men who reported occasional cocaine use (n = 6) and in men who met DSM-III-R diagnostic criteria for dependence on both cocaine and opiates (n = 6). Peak plasma cocaine levels were equivalent in the two groups, and pharmacokinetic analyses revealed no significant differences in cocaine levels at any time. Cocaine induced a significantly greater increase in ACTH in the occasional cocaine users than in the cocaine dependent men (p < .01). Heart rate and systolic and diastolic blood pressure increases after cocaine were also significantly greater in the occasional cocaine users than in the cocaine-dependent men (p < .05). These neuroendocrine and physiologic differences were paralleled by significantly greater subjective reports of “high” and “euphoria” by the occasional cocaine users (p < .03 to .0001). These data are consistent with the conclusion that tolerance to cocaine's physiologic, neuroendocrine, and subjective effects may occur as a function of chronic use.
Similar content being viewed by others
Main
There is considerable evidence that administration of cocaine induces acute tolerance for behavioral and cardiovascular effects in humans (Ambre et al. 1988; Chow et al. 1985; Fischman et al. 1983, 1985; Foltin and Fischman 1991, 1992). However, acute tolerance for cocaine's behavioral and cardiovascular responses was not observed when an initial acute bolus dose of cocaine was followed by continuous cocaine infusions for 12 to 240 min (Kumor et al. 1989). It has been postulated that chronic administration may result in a decrement in cocaine-induced elevations of blood pressure, reflecting tolerance to cocaine's effects (Jaffe 1985). Behavioral tolerance has been observed in experimental animals during a 14-day period of continuous cocaine administration (King et al. 1994). Chronic tolerance for cocaine's local anesthetic effects has also been reported in preclinical studies (Castellani et al. 1978). Tolerance for cocaine at doses that induce lethal intoxication in naive animals may also occur as a consequence of chronic cocaine administration (Ellenhorn and Barceloux 1988). One clinical case report of cocaine tolerance was believed to have occurred as a concomitant of long-term cocaine self-administration (Howell and Ezell 1990).
A major criterion for the diagnosis of substance dependence (including cocaine dependence) specified in the Diagnostic and Statistical Manual of Mental Disorders (1994) is as follows: “1. Tolerance, as defined by either of the following: a. A need for markedly increased amounts of the substance to achieve intoxication or desired effect; b. Markedly diminished effect with continued use of the same amount of the substance.” We have been unable to locate any controlled studies to determine if chronic cocaine use is associated with tolerance for cocaine's behavioral, cardiovascular, and neuroendocrine effects in men. The purpose of this study was to compare the acute effects of cocaine in two groups of subjects with different histories of cocaine use; occasional cocaine users and men who fulfilled the diagnostic criteria (DSM-III-R) for cocaine dependence and dual dependence on opiates. We postulated that persons with a long-term history of cocaine abuse might be less responsive to the effects of an acute cocaine challenge than occasional users. If significant differences in responsivity occurred after a controlled period of cocaine abstinence, this would be consistent with the notion that chronic cocaine exposure induces tolerance to some of its effects.
METHODS
Subjects
Twelve adult men between the ages of 21 and 35 provided informed consent for participation in studies to determine the acute effects of cocaine on behavioral, cardiovascular, and neuroendocrine function. Six men between the ages of 26 to 35 with a mean weight of 77.4 kg met DSM-III-R axis I diagnostic criteria for concurrent opioid and cocaine dependence. Six men between the ages of 21 and 32 years reported occasional cocaine use. There were no statistically significant differences between the two groups with respect to weight, height, and body mass index (BMI). Subject characteristics are summarized in Table 1.
Men diagnosed with concurrent opioid and cocaine dependence reported an average of 13 years of chronic intravenous opiate use and 9 years of chronic cocaine use. These men stated that they occasionally used speedballs, i.e., simultaneous intravenous injections of cocaine and heroin, but usually they self-administered heroin before or after intravenous injection of cocaine. In contrast, occasional cocaine users reported using cocaine by inhalation and insufflation on five to 10 occasions per year during the year before the study. All men (occasional cocaine users and those with concurrent heroin and cocaine dependence) were in good physical health, had normal medical and laboratory screening examinations, and provided informed consent for participation in the studies. All subjects were drug-free at the time of the study as assessed by urine-drug screens. Men with concurrent heroin and cocaine dependence participated in a study to determine the safety and effectiveness of buprenorphine for the treatment of their drug-dependence disorder. A report of these studies in 20 subjects has been described previously (Teoh et al. 1993). The subset of six subjects described in the present report were selected on the basis of their comparability to the occasional cocaine users with respect to age, weight, height, body mass index (BMI), and the intravenous dose of cocaine administered in the study.
Experimental Procedures
The cocaine- and opioid-dependent men resided on a clinical research ward during methadone detoxification. After the conclusion of methadone detoxification, men were drug-free for 9 days. On study days 7, 8, or 9, men were given an intravenous challenge dose of 0.39 ± 0.02 mg/kg cocaine intravenously over a 1-min interval. Cocaine injections were administered by a physician. All men were studied in a semisupine position, and continuous cardiovascular monitoring was carried out for 10 min before intravenous cocaine administration and for 2 h after drug injection. A trained physician certified for cardiopulmonary resuscitation was present during each study, and a cardiac defibrillator and appropriate emergency treatment medications were located in the study room.
The occasional cocaine users also were studied on a clinical research ward. Identical procedures for intravenous cocaine administration were used in occasional cocaine users and in men who were heroin and cocaine dependent. A challenge dose of cocaine (0.4 mg/kg) was administered intravenously over a 1-min period.
Subjects were asked to report their perception of drug intensity and drug quality (euphoria) 5 min after completion of intravenous cocaine injection. Measurements of heart rate and systolic and diastolic blood pressure were recorded before intravenous cocaine injection and at +5, +10, +15, +20, +30, +45, +60, +90 and +120 min in all subjects. Blood samples for determination of plasma cocaine levels and adrenocorticotropic hormone (ACTH) were obtained at baseline and at +5, +15, +30, +45, +60, +90 and +120 min after intravenous cocaine injection. All plasma samples for cocaine and ACTH analyses were obtained from an intravenous catheter in the arm opposite the arm into which cocaine was intravenously injected.
Cocaine Hydrochloride and Placebo Preparation
Cocaine hydrochloride was acquired from the National Institute of Drug Abuse in powder form and was dissolved in sterile water for intravenous injection. Sterility was ensured by passing the solution through a 0.22-micron millipore filter and subjecting it to a Limulus Amebocyte Lysate (LAL) test for detection of gram negative bacterial endotoxins. The test kit was manufactured by Whittaker Bioproducts (Walkersville, MD). Commercial preparation of 0.9% saline (1 ml) in sterile vials was used as the placebo challenge.
ACTH Radioimmunoassay Procedures
Plasma ACTH concentrations were measured in duplicate using an immunoradiometric assay (IRMA) kit purchased from Nichols Institute Diagnostics (Allegro, CA). The assay sensitivity was 0.15 pmol/L and the intra- and interassay coefficients of variance were 2.9% and 8.2%, respectively.
Plasma Cocaine Analysis
Plasma cocaine levels were measured in duplicate using a liquid/liquid extraction method described by Jacob (Jacob et al. 1987) with a Hewlet-Packard Model 5890A gas chromatograph equipped with a capillary column and a Nitrogen-Phosphorous detector. The assay sensitivity was 6.5 ng/ml and the intra-assay coefficient of variance was 2.2%.
Data Analysis
Subjective responses were analyzed with a one-way analysis of variance (ANOVA). Plasma ACTH and cocaine values for subjects were analyzed using a 2 (group) × 8 (time) repeated measures ANOVA. If significant main effects were detected, one-way ANOVAs were performed to identify the times at which groups significantly differed. Heart rate, systolic blood pressure, and diastolic blood pressure were evaluated by similar procedures. Cocaine pharmacokinetics were analyzed with a pharmacologic calculation system based upon the Manual of Pharmacologic Calculations with Computer Programs, 2nd ed. (Tallarida and Murray 1991), using PHARM/PCS Version 4.2, MicroComputer Specialists (MCS), Philadelphia, PA 19106.
RESULTS
Plasma cocaine levels in the cocaine-and opiate-dependent men and the occasional cocaine users are shown in Figure 1 . Peak plasma cocaine levels exceeded 200 ng/ml in both groups. Cocaine pharmacokinetics are presented in Table 2. There were no significant differences in plasma cocaine levels in the cocaine-opiate–dependent men and occasional users at any time.
ACTH levels before and after placebo and cocaine administration are shown in Figure 2 . There were no significant differences in baseline plasma ACTH levels before intravenous placebo or cocaine administration. There were no significant placebo-induced changes in ACTH levels. ACTH levels in the cocaine- and opiate- dependent men were significantly lower than in occasional cocaine users (p < .0113) at time points 5, 15, 30, 45, and 120 min after intravenous cocaine administration.
Cardiovascular effects of cocaine and placebo administration (heart rate, systolic blood pressure, and diastolic blood pressure) in the cocaine- and opiate-dependent men and the occasional cocaine users are shown in Figure 3 . There were no significant changes in heart rate and blood pressure after intravenous placebo in either group. Cocaine-induced increases in heart rate, systolic blood pressure, and diastolic blood pressure were significantly greater in the occasional cocaine users than in the cocaine- and opiate-dependent men (p < 0.05).
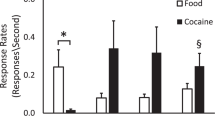
Subjective responses of the cocaine- and opiate-dependent men and the occasional cocaine users at 5 min after completion of intravenous placebo and cocaine administration are shown in Figure 4 . No statistically significant changes were reported after intravenous placebo in either group. Occasional cocaine users reported a significantly greater perception of “high” (p = .03) as well as significantly greater euphoria (p < .0001) 5 min after cocaine administration.
DISCUSSION
There is considerable clinical evidence that people who abuse or who are dependent upon cocaine “report requiring more cocaine over time to obtain euphoria, i.e., tolerance” (O'Brien 1996). However, the current study is the first controlled assessment of persistent behavioral, cardiovascular, and neuroendocrine tolerance for cocaine during a period of cocaine abstinence. Cocaine-induced increases in ACTH, cardiovascular, and subjective effect measures were significantly greater in the occasional cocaine users than in the cocaine-dependent men. These differences occurred even though peak plasma cocaine levels, and the rate of decrement in plasma cocaine levels after intravenous drug administration did not differ significantly between the two groups. The computed half-life of cocaine levels in plasma in both the occasional cocaine users (49 ± 3 min) and the cocaine-dependent men (46 ± 2.5 min) was virtually identical to data (48 ± 13 min) reported by Chow and co-workers (Chow et al. 1985). Subjects who participated in the research carried out by Chow and co-workers (Chow et al. 1985) were four men and one woman who reported cocaine use at least one time per week for 2 months before the study. The cocaine-dependent men described in this report had been drug-free for 9 days after admission to the clinical research ward and before administration of the cocaine challenge dose. These data further suggest that prolonged cocaine exposure may be associated with persistent alterations in physiologic and subjective responses to cocaine without significant changes in cocaine pharmacokinetics. These data suggest that cocaine may induce prolonged neurobiologic changes in chronic users.
The occasional cocaine users were also drug-free prior to the study, and this was verified by urine drug screens. Consequently it was unlikely that recent cocaine use could account for an enhanced responsivity to cocaine's physiologic and behavioral effects in these men. Sensitization to cocaine's effects has been reported in experimental animal studies after administration of cocaine and other stimulants (Cunningham et al. 1992; Kalivas et al. 1992; Post and Weiss 1988; Post et al. 1988; Stripling and Ellinwood 1977; Unterwald et al. 1994; Zahniser et al. 1988), but sensitization to drug-induced euphoria or cardiovascular responsivity has not been observed in humans (O'Brien 1996). Because our occasional cocaine users reported using cocaine quite infrequently, it is unlikely that an antecedent cocaine-induced sensitization could explain the results obtained. Ethical and medical considerations precluded administration of intravenous cocaine to drug-naive individuals.
Cocaine and Opiate Interactions
It is also possible that the preexisting condition of dual dependence on both opioids and cocaine contributed to the results observed. There is considerable clinical evidence that cocaine dependence is often associated with concurrent opiate abuse and dependence (Condelli et al 1991; Gastfriend et al. 1993; Kosten et al. 1989, 1987; Schottenfeld et al. 1993). Moreover, controlled clinical studies report that methadone maintenance may enhance the subjective effects of cocaine in experienced users under some conditions (Foltin et al. 1995; Preston et al. 1996). Although opiate dependence in a drug-free individual (as in the present study) and concurrent maintenance on an opiate (as in the studies by Foltin et al. 1995; Preston et al. 1996) are not equivalent conditions, these clinical data are more consistent with the possibility that opioid exposure may increase, rather than decrease, the acute effects of cocaine. Preclinical studies often indicate that opiates increase, rather than decrease, the effects of cocaine on some behavioral endpoints. For example, pretreatment with mu receptor selective opioids potentiated cocaine's discriminative stimulus effects in squirrel monkeys (Spealman and Bergman 1992, 1994). Similarly, in rhesus monkeys, pretreatment with the mu opioid agonist fentanyl and morphine increased cocaine's discriminative stimulus effects in some monkeys (Negus et al., in press).
Experimental studies in rodents have demonstrated that opiate administration may induce locomotor sensitization after administration of cocaine or amphetamines (DuMars et al. 1988; Kalivas 1985; Vezina et al. 1989; Vezina and Stewart 1990). In addition, opiate administration before cocaine administration may facilitate rather than inhibit cocaine-conditioned place preferences (Bilsky et al. 1992) and conditioned reinforcement (Cunningham and Kelley 1992). One important mechanisms underlying opiate-cocaine cross sensitization may be related to the effects of both drugs on the cyclic adenosine monophosphate AMP system (Cunningham et al. 1997). Because men who were concurrently dependent upon heroin and cocaine had significantly lower cardiovascular and neuroendocrine responses to intravenous cocaine administration than the occasional cocaine users, opiate-induced sensitization to cocaine effects is unlikely to account for the different responses observed in the current study. The significant differences in cocaine-induced increments in plasma ACTH levels in occasional cocaine users and cocaine-dependent men were paralleled by statistically significantly greater increments in heart rate and diastolic blood pressure and the perception of “high” and “euphoria” in the occasional cocaine users than in cocaine and opiate-dependent men.
Cocaine and ACTH Interactions
It has been postulated that cocaine-related stimulation of corticotrophin releasing factor (CRF) may be one mechanism underlying the reinforcing properties of cocaine in experimental animals and humans (Borowsky and Kuhn 1991b; Calogero et al. 1989; Levy et al. 1991; Mendelson et al. 1992a, b; Moldow and Fischman 1987; Rivier and Vale 1987; Sarnyai et al. 1992; Teoh et al. 1994; Vescovi et al. 1992). Several studies that have assessed sensitization and tolerance associated with chronic cocaine administration to rodents did not reveal any significant degree of tolerance for cocaine-induced stimulation of ACTH. Borowsky and Kuhn found no changes in cocaine-stimulation of ACTH after 3 and 7 days of chronic cocaine administration to rats (Borowsky and Kuhn 1991a). Levy et al. (1992) also did not observe any changes in ACTH stimulation after cocaine administration for 14 days. The differences observed in this study between occasional cocaine users and men with concurrent cocaine and opiate dependence and data obtained with rodents may reflect species differences. However, the cocaine-dependent men who participated in this study reported a very long duration (mean 8.83 years) of heavy cocaine use. It is possible that chronic cocaine administration for up to 14 days may not be long enough to result in tolerance for cocaine-induced ACTH stimulation in rodents.
There is evidence that synthetic CRF administration has a number of cocaine-like effects, including induction of stereotyped behaviors and increased locomotor activity (Dunn and Berridge 1990). Recent studies have demonstrated that some cocaine-induced changes in the behavior of rodents can be modulated by blockade of corticosterone secretion (Marinelli et al. 1997). Moreover, adrenalectomy completely eliminated cocaine self-administration in rats (Goeders and Guerin 1996), a finding consistent with the notion that ACTH (and by inference CRF) may be important for cocaine's reinforcing effects. We have previously observed that maintenance on buprenorphine, an opioid mixed agonist-antagonist, suppressed both cocaine-induced euphoria and ACTH secretion in humans (Mendelson et al. 1992a). Buprenorphine also reduced cocaine self-administration by rhesus monkeys (Mello and Mendelson 1993, 1995; Mello et al. 1989, 1990). The current observations that cocaine induces greater increments in ACTH secretion and greater behavioral and cardiovascular responses in occasional cocaine users than in men with a past history of cocaine and heroin dependence suggests that there is a relationship between neuroendocrine, cardiovascular, and behavioral tolerance for cocaine in humans.
References
Ambre JJ, Belknap SM, Nelson J, Ruo TI, Shin S, Atkinson AJ . (1988): Acute tolerance to cocaine in humans. Clin Pharmacol Ther 44: 1–8
American Psychiatric Association. (1994): Diagnostic and Statistical Manual of Mental Disorders, 4th ed. DSM-IV. Washington, DC, American Psychiatric Association
Bilsky EJ, Montegut MJ, Delong CL, Reid LD . (1992): Opioidergic modulation of cocaine conditioned place preferences. Life Sci 50: PL85–PL90
Borowsky B, Kuhn CM . (1991a): Chronic cocaine administration sensitizes behavioral but not neuroendocrine responses. Brain Res 543: 301–306
Borowsky B, Kuhn CM . (1991b): Monoamine mediation of cocaine-induced hypothalamo-pituitary-adrenal activation. J Pharmacol Exp Ther 256: 204–210
Calogero AE, Gallucci WT, Kling MA, Chrousos GP, Gold PW . (1989): Cocaine stimulates rat hypothalamic corticotropin-releasing hormone secretion in vitro. Brain Res 505: 7–11
Castellani S, Ellinwood E, Kilbey M . (1978): Behavioral analysis of chronic cocaine intoxication in the cat. Biol Psychiatry 13: 203–215
Chow MJ, Ambre JJ, Ruo TI, Atkinson AJ, Bowsher DJ, Fischman MW . (1985): Kinetics of cocaine distribution, elimination, and chronotropic effects. Clin Pharm Ther 38: 318–324
Condelli WS, Fairbank JA, Dennis ML, Rachal JV . (1991): Cocaine use by clients in methadone programs: Significance, scope, and behavioral interventions. J Subst Abuse Treat 8: 203–212
Cunningham KA, Paris JM, Goeders NE . (1992): Serotonin neurotransmission in cocaine sensitization. Ann NY Acad Sci 654: 117–127
Cunningham ST, Finn M, Kelley AE . (1997): Sensitization of the locomotor response to psychostimulants after repeated opiate exposure: Role of the nucleus accumbens. Neuropsychopharmacology 16: 147–155
Cunningham ST, Kelley AE . (1992): Evidence for opiate-dopamine cross-sensitization in nucleus accumbens: Studies of conditioned reward. Brain Res Bull 29: 675–680
DuMars LA, Rodger LD, Kalivas PW . (1988): Behavioral cross-sensitization between cocaine and enkephalin in the A10 dopamine region. Behav Brain Res 27: 87–91
Dunn AJ, Berridge CW . (1990: Physiological and behavioral responses to corticotropin-releasing factor administration: Is CRF a mediator of anxiety or stress responses. Brain Res Rev 15: 71–100
Ellenhorn MJ, Barceloux DG . (1988): Medical Toxicology. New York, Elsevier, pp 643–647
Fischman MW, Schuster CR, Hatano Y . (1983): A comparison of the subjective and cardiovascular effects of cocaine and lidocaine in humans. Pharmacol Biochem Behav 18: 123–127
Fischman MW, Schuster CR, Javaid JK, Hatono Y, Davis J . (1985): Acute tolerance development to the cardiovascular and subjective effects of cocaine. J Pharmacol Exp Ther 235: 677–682
Foltin RW, Christiansen I, Levin FR, Fischman MW . (1995): Effects of single and multiple intravenous cocaine injections in humans maintained on methadone. J Pharmacol Exp Ther 275: 38–47
Foltin RW, Fischman MW . (1991): Smoked and intravenous cocaine in humans: Acute tolerance, cardiovascular and subjective effects. J Pharmacol Exp Ther 257: 247–261
Foltin RW, Fischman MW . (1992): Self-administration of cocaine by humans: Choice between smoked and intravenous cocaine. J Pharmacol Exp Ther 231: 841–849
Gastfriend DR, Mendelson JH, Mello NK, Teoh SK, Reif S . (1993): Buprenorphine pharmacotherapy for concurrent heroin and cocaine dependence. Am J Addict 2: 269–278
Goeders NE, Guerin GF . (1996): Effects of surgical and pharmacological adrenalectomy on the initiation and maintenance of intravenous cocaine self-administration in rats. Brain Res 722: 145–152
Howell SL, Ezell AL . (1990): An example of cocaine tolerance in a gunshot wound fatality. J Anal Toxicol 14: 6–7
Jacob P, Elias-Baker GA, Jones RT, Benowitz NL . (1987): Determination of benzoylecgonine and cocaine in biologic fluids by automated gas chromatography. J Chromatogr 417: 277–286
Jaffe J . (1985): Drug addiction and drug abuse. In Goodman AG, Goodman LS, Rall TW, Murad F (eds), The Pharmacological Basis of Therapeutics. New York, Macmillan, pp 535–584
Kalivas PW . (1985): Interactions between neuropeptides and dopamineneurons in the ventromedial mesencephalon. Neurosci Biobehav Rev 9: 573–587
Kalivas PW, Striplin CD, Steketee JD, Klitenick MA, Duffy P . (1992): Cellular mechanisms of behavioral sensitization to drugs of abuse. Ann NY Acad Sci 654: 128–135
King GR, Ellinwood EH Jr, Silvia C, Joyner CM, Xue Z, Caron M, Lee TH . (1994): Withdrawal from continuous or intermittent cocaine I. Changes in D2 receptor function. J Pharmacol Exp Ther 269: 743–749
Kosten TR, Kleber HD, Morgan C . (1989): Role of opioid antagonists in treating intravenous cocaine abuse. Life Sci 44: 887–892
Kosten TR, Rounsaville BJ, Gawin FH, Kleber HD . (1987): A 2.5-year follow-up of cocaine use among treated opioid addicts. Arch Gen Psychiatry 44: 281–284
Kumor KM, Sherer MA, Cascella NG . (1989): Cocaine use in man: Subjective effects, physiologic responses, and toxicity. In Redda KK, Walker CA, Barnett G (eds), Cocaine, Marijuana, Designer Drugs: Chemistry, Pharmacology and Behavior. Boca Raton, FL, CRC Press, Inc., pp 83–96
Levy AD, Li Q, Alvarez Sanz MC, Rittenhouse PA, Kerr JE, Van de Kar LD . (1992): Neuroendocrine responses to cocaine do not exhibit sensitization following repeated cocaine exposure. Life Sci 51: 887–897
Levy AD, Li Q, Kerr JE, Rittenhouse PA, Milonas G, Cabrera TM, Battaglia G, Alvarez Sanz MC, Van de Kar LD . (1991): Cocaine-induced elevation of plasma adrenocorticotropin hormone and corticosterone is mediated by serotonergic neurons. J Pharmacol Exp Ther 259: 495–500
Marinelli M, Route-Pont F, DeJesus-Oliveira C, LeMoal M, Piazza PV . (1997): Acute blockade of corticosterone secretion decreases the psychomotor stimulant effects of cocaine. Neuropsychopharmacology 16: 156–161
Mello NK, Mendelson JH . (1993): Buprenorphine's effects on cocaine and heroin abuse. In Korenman S and Barcus J (eds), The Biological Basis of Substance Abuse. New York, Oxford University Press, pp 463–485
Mello NK, Mendelson JH . (1995): Buprenorphine treatment of cocaine and heroin abuse. In Cowan A, Lewis JW (ed), Buprenorphine: Combatting Drug Abuse with a Unique Opioid. New York, Wiley-Liss, Inc., pp 241–287
Mello NK, Mendelson JH, Bree MP, Lukas S . (1990): Buprenorphine and naltrexone effects on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther 254: 926–939
Mello NK, Mendelson JH, Bree MP, Lukas SE . (1989): Buprenorphine suppresses cocaine self-administration by rhesus monkey. Science 245: 859–862
Mendelson JH, Teoh SK, Mello NK, Ellingboe J . (1992a): Buprenorphine attenuates the effects of cocaine on adrenocorticotrophin (ACTH) secretion and mood states in man. Neuropsychopharmacology 17: 157–162
Mendelson JH, Teoh SK, Mello NK, Ellingboe J, Rhoades E . (1992b): Acute effects of cocaine on plasma adrenocorticotropic hormones, luteinizing hormone and prolactin levels in cocaine-dependent men. J Pharmacol Exp Ther 263: 505–509
Moldow RL, Fischman AJ . (1987): Cocaine induced secretion of ACTH, beta-endorphin, and corticosterone. Peptides 8: 819–822
Negus SS, Gatch MB, Mello NK . (1997): Effects of mu opioid agonists alone and in combination with cocaine and d-amphetamine in rhesus monkeys trained to discriminate cocaine. Neuropsychopharmacology (in press).
O'Brien CP . (1996): Drug addiction and drug abuse. In Goodman G and Gilman G (eds), The Pharmacological Basis of Therapeutics, 9th ed. New York, McGraw-Hill, pp 557–559
Post RM, Weiss SR . (1988): Psychomotor stimulant vs. local anesthetic effects of cocaine: Role of behavioral sensitization and kindling. NIDA Res Monogr 88: 217–238
Post RM, Weiss SR, Pert A . (1988): Implications of behavioral sensitization and kindling for stress-induced behavioral change. Adv Exp Med Biol 245: 441–463
Preston KL, Sullivan JT, Strain EC, Bigelow GE . (1996): Enhancement of cocaine's abuse liability in methadone maintained patients. Psychopharmacology 123: 15–25
Rivier C, Vale W . (1987): Cocaine stimulates adrenocorticotropin (ACTH) secretion through a corticotropin-releasing factor (CRF)-mediated mechanism. Brain Res 422: 403–406
Sarnyai Z, Biro E, Penke B, Telegdy G . (1992): The cocaine-induced elevation of plasma corticosterone is mediated by endogenous corticotropin-releasing factor (CRF) in rats. Brain Res 589: 154–156
Schottenfeld RS, Pakes J, Ziedonis D, Kosten TR . (1993): Buprenorphine: Dose-related effects on cocaine and opioid use in cocaine-abusing opioid-dependent humans. Biol Psychiatry 34: 66–74
Spealman RD, Bergman J . (1992): Modulation of the discriminative stimulus effects of cocaine by mu and kappa opioids. J Pharmacol Exp Ther 261: 607–615
Spealman RD, Bergman J . (1994): Opioid modulation of the discriminative stimulus effects of cocaine: Comparison of μ, κ, and δ agonists in squirrel monkeys discriminating low doses of cocaine. Behav Pharmacol 5: 21–31
Stripling JS, Ellinwood EH Jr . (1977): Sensitization to cocaine following chronic administration in the rat. In Ellinwood Jr EH, Kilbey MD (eds), Cocaine and Other Stimulants. New York, Plenum Press, pp 327–351
Tallarida RJ, Murray RB . (1991): Manual of Pharmacologic Calculations with Computer Programs, 2nd ed. New York, Springer-Verlag
Teoh SK, Mendelson JH, Mello NK, Kuehnle J, Sintavanarong P, Rhoades EM . (1993): Acute interactions of buprenorphine with intravenous cocaine and morphine: An investigational new drug phase I safety evaluation. J Clin Psychopharmacol 13: 87–99
Teoh SK, Sarnyai Z, Mendelson JH, Mello NK, Springer SA, Sholar JW, Wapler M, Gelles H . (1994): Cocaine effects on pulsatile secretion of ACTH in men. J Pharmacol Exp Ther 270: 1134–1138
Unterwald EM, Ho A, Rubenfeld JM, Kreek MJ . (19940: Time course of the development of behavioral sensitization and dopamine receptor up-regulation during binge cocaine administration. J Pharmacol Exp Ther 270: 1387–1396
Vescovi PP, Coivo V, Volpi R, Passeri M . (1992): Diurnal variations in plasma ACTH, cortisol and beta-endorphin levels in cocaine addicts. Horm Res 37: 221–224
Vezina P, Giovino AA, Wise RA, Stewart J . (1989): Environment-specific cross-sensitization between the locomotor activating effects of morphine and amphetamine. Pharmacol Biochem Behav 32: 581–584
Vezina P, Stewart J . (1990: Amphetamine administered to the ventral tegmental area but not to the nucleus accumbens sensitizes rats to systemic morphine: Lack of conditioned effects. Brain Res 516: 99–106
Zahniser NR, Peris J, Dwoskin LP, Curella P, Uasuda RP, O'Keefe L, Boyson SJ . (1988): Sensitization to cocaine in the nigrostriatal dopamine system. NIDA Res Monogr 88: 55–77
Acknowledgements
This research was supported in part by grants DA 04059, DA 06116, DA 00064, and DA 00101 from the National Institute on Drug Abuse, National Institutes of Health, Bethesda, MD. We are grateful to Gloria Yong Hong Cheng and Howard Gelles for analysis of plasma cocaine and ACTH samples. The six cocaine- and opiate-dependent men described in the present report were selected from a larger sample of 20 men to match characteristics of the occasional cocaine users. Group data for the 20 cocaine- and opiate-dependent men were previously reported in Teoh et al. (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mendelson, J., Sholar, M., Mello, N. et al. Cocaine Tolerance: Behavioral, Cardiovascular, and Neuroendocrine Function in Men. Neuropsychopharmacol 18, 263–271 (1998). https://doi.org/10.1016/S0893-133X(97)00146-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1016/S0893-133X(97)00146-2
Keywords
This article is cited by
-
Molecular pattern of a decrease in the rewarding effect of cocaine after an escalating-dose drug regimen
Pharmacological Reports (2023)
-
Sex and drug differences in stress, craving and cortisol response to the trier social stress task
Psychopharmacology (2022)
-
The Effect of Mandatory Play Breaks on Subsequent Gambling Behavior Among Norwegian Online Sports Betting, Slots and Bingo Players: A Large-scale Real World Study
Journal of Gambling Studies (2021)
-
Effects of cocaine on the hypothalamic–pituitary–adrenal axis
Journal of Endocrinological Investigation (2014)
-
Temporal Pattern of Cocaine Intake Determines Tolerance vs Sensitization of Cocaine Effects at the Dopamine Transporter
Neuropsychopharmacology (2013)