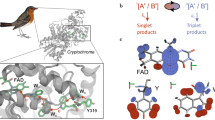
Abstract
Recent evidence suggests that a variety of organisms may harness some of the unique features of quantum mechanics to gain a biological advantage. These features go beyond trivial quantum effects and may include harnessing quantum coherence on physiologically important timescales. In this brief review we summarize the latest results for non-trivial quantum effects in photosynthetic light harvesting, avian magnetoreception and several other candidates for functional quantum biology. We present both the evidence for and arguments against there being a functional role for quantum coherence in these systems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Schrödinger, E. What is Life? (Cambridge Univ. Press, 1992).
Davies, P. C. W. Quantum Aspects of Life (Imperial College Press, 2008).
Longuet-Higgins, H. C. Quantum mechanics and biology. Biophys. J. 2, 207–215 (1962).
Wolynes, P. G. Some quantum weirdness in physiology. Proc. Natl Acad. Sci. USA 106, 17247–17248 (2009).
Tegmark, M. Importance of quantum decoherence in brain processes. Phys. Rev. E 61, 4194–4206 (2000).
McKemmish, L. K., Reimers, J. R., McKenzie, R. H., Mark, A. E. & Hush, N. S. Penrose–Hameroff orchestrated objective-reduction proposal for human consciousness is not biologically feasible. Phys. Rev. E 80, 021912 (2009).
Maeda, K. et al. Magnetically sensitive light-induced reactions in cryptochrome are consistent with its proposed role as a magnetoreceptor. Proc. Natl Acad. Sci. USA 109, 4774–4779 (2012).
Van Amerongen, H., Valkunas, L. & van Grondelle, R. Photosynthetic excitons (World Scientific, 2000).
Blankenship, R. E. Molecular Mechanisms of Photosynthesis (Blackwell Science, 2002).
Cogdell, R. J., Gardiner, A. T., Hashimoto, H. & Brotosudarmo, T. H. P. A comparative look at the first few milliseconds of the light reactions of photosynthesis. Photochem. Photobiol. Sci. 7, 1150–1158 (2008).
Cogdell, R. J., Gall, A. & Köhler, J. The architecture and function of the light-harvesting apparatus of purple bacteria: From single molecules to in vivo membranes. Quart. Rev. Biophys. 39, 227–324 (2006).
Engel, G. S. et al. Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems. Nature 446, 782–786 (2007).
Ishizaki, A., Calhoun, T. R., Schlau-Cohen, G. S. & Fleming, G. R. Quantum coherence and its interplay with protein environments in photosynthetic electronic energy transfer. Phys. Chem. Chem. Phys. 12, 7319–7337 (2010).
Lee, H., Cheng, Y-C. & Fleming, G. R. Coherence dynamics in photosynthesis: Protein protection of excitonic coherence. Science 316, 1462–1465 (2007).
Collini, E. et al. Coherently wired light-harvesting in photosynthetic marine algae at ambient temperature. Nature 463, 644–648 (2010).
Panitchayangkoon, G. et al. Long-lived quantum coherence in photosynthetic complexes at physiological temperature. Proc. Natl Acad. Sci. USA 107, 12766–12770 (2010).
Panitchayangkoon, G. et al. Direct evidence of quantum transport in photosynthetic light-harvesting complexes. Proc. Natl Acad. Sci. USA 108, 20908–20912 (2011).
De Liberato, S. & Ueda, M. Carnot’s theorem for nonthermal stationary reservoirs. Phys. Rev. E 84, 051122 (2011).
Ishizaki, A. & Fleming, G. R. Theoretical examination of quantum coherence in a photosynthetic system at physiological temperature. Proc. Natl Acad. Sci. USA 106, 17255–17260 (2009).
Yen, T-C. & Cheng, Y-C. Electronic coherence effects in photosynthetic light harvesting. Proc. Chem. 3, 211–221 (2011).
Fleming, G. R., Scholes, G. D. & Cheng, Y-C. Quantum effects in biology. Proc. Chem. 3, 38–57 (2011).
Scholes, G. D., Fleming, G. R., Olaya-Castro, A. & van Grondelle, R. Lessons from nature about solar light harvesting. Nature Chem. 3, 763–774 (2011).
Cheng, Y-C. & Fleming, G. R. Dynamics of light harvesting in photosynthesis. Annu. Rev. Phys. Chem. 60, 241–262 (2009).
Renger, T. Theory of excitation energy transfer: From structure to function. Photosynth. Res. 102, 471–485 (2009).
Adolphs, J. & Renger, T. How proteins trigger excitation energy transfer in the FMO complex of green sulphur bacteria. Biophysical J. 91, 2778–2797 (2006).
Plenio, M. B. & Huelga, S. F. Dephasing-assisted transport: Quantum networks and biomolecules. New J. Phys. 10, 113019 (2008).
Mohseni, M., Robentrost, P., Lloyd, S. & Aspuru-Guzik, A. Environment-assisted quantum walks in photosynthetic energy transfer. J. Chem. Phys. 129, 176106 (2008).
Gaab, K. M. & Bardeen, C. J. The effects of connectivity, coherence, and trapping on energy transfer in simple light-harvesting systems studied using the Haken–Strobl model with diagonal disorder. J. Chem. Phys. 121, 7813–7820 (2004).
Lee, H., Cheng, Y-C. & Fleming, G. R. Quantum coherence accelerating photosynthetic energy transfer. Springer Ser. Chem. Phys. 92, 607–609 (2009).
Rebentrost, P., Mohseni, M., Kassal, I., Lloyd, S. & Aspuru-Guzik, A. Environment-assisted quantum transport. New J. Phys. 11, 033003 (2009).
Ghosh, P. K., Smirnov, A. Y. & Nori, F. Quantum effects in energy and charge transfer in an artificial photosynthetic complex. J. Chem. Phys. 134, 244103 (2011).
Cheng, Y-C., Engel, G. S. & Fleming, G. R. Elucidation of population and coherence dynamics using cross-peaks in two-dimensional electronic spectroscopy. Chem. Phys. 341, 285–295 (2007).
Jang, S. Theory of multichromophoric coherent resonance energy transfer: A polaronic quantum master equation approach. J. Chem. Phys. 135, 034105 (2011).
Jang, S., Cheng, Y-C., Reichman, D. R. & Eaves, J. D. Theory of coherent resonance energy transfer. J. Chem. Phys. 129, 101104 (2008).
Nalbach, P., Ishizaki, A., Fleming, G. R. & Thorwart, M. Iterative path-integral algorithm versus cumulant time-nonlocal master equation approach for dissipative biomolecular exciton transport. New J. Phys. 13, 063040 (2011).
Ritschel, G., Roden, J., Strunz, W. T., Aspuru-Guzik, A. & Eisfeld, A. On the suppression of quantum oscillations and the choice of site energies in electronic excitation transfer in the Fenna–Matthews–Olson trimer. J. Phys. Chem. Lett. 2, 2912–2917 (2011).
Ghosh, P., Smirnov, A. & Nori, F. Artificial photosynthetic reaction centers coupled to light-harvesting antennas. Phys. Rev. E 84, 061138 (2011).
Kolli, A., Nazir, A. & Olaya-Castro, A. Electronic excitation dynamics in multichromophoric systems described via a polaron-representation master equation. J. Chem. Phys. 135, 154112 (2011).
Wu, J., Liu, F., Shen, Y., Cao, J. & Silbey, R. J. Efficient energy transfer in light-harvesting systems, I: Optimal temperature, reorganization energy, and spatial-temporal correlations. New J. Phys. 12, 105012 (2010).
Christensson, N., Kauffmann, H. F., Pullerits, T. & Mančal, T. Origin of long-lived coherences in light-harvesting complexes. J. Phys. Chem. B 116, 7449–7454 (2012).
Mančal, T. et al. System-dependent signatures of electronic and vibrational coherences in electronic two-dimensional spectra. J. Phys. Chem. Lett. 3, 1497–1502 (2012).
Hayes, D., Wen, J., Panitchayangkoon, G., Blankenship, R. E. & Engel, G. S. Robustness of electronic coherence in the Fenna–Matthews–Olson complex to vibronic and structural modifications. Faraday Discuss. 150, 459–469 (2011).
Olbrich, C., Strumpfer, J., Schulten, K. & Kleinekathofer, U. Quest for spatially correlated fluctuations in the FMO light-harvesting complex. J. Phys. Chem. B 115, 758–764 (2011).
Jing, Y., Zheng, R., Li, H-X. & Shi, Q. Theoretical study of the electronic-vibrational coupling in the Q(y) states of the photosynthetic reaction center in purple bacteria. J. Phys. Chem. B 116, 1164–1171 (2012).
Wu, J., Liu, F., Ma, J., Silbey, R. J. & Cao, J. Efficient energy transfer in light-harvesting systems, II: Quantum-classical comparison, flux network, and robustness analysis. Preprint at http://arxiv.org/abs/1109.5769 (2011).
Briggs, J. S. & Eisfeld, A. Equivalence of quantum and classical coherence in electronic energy transfer. Phys. Rev. E 83, 051911–051914 (2011).
Miller, W. H. Perspective: Quantum or classical coherence? J. Chem. Phys. 136, 210901 (2012).
Wilde, M. M., McCracken, J. M. & Mizel, A. Could light harvesting complexes exhibit non-classical effects at room temperature? Proc. R. Soc. A 446, 1347–1363 (2010).
Li, C-M., Lambert, N., Chen, Y-N., Chen, G-Y. & Nori, F. Witnessing quantum coherence: From solid-state to biological systems. Sci. Rep. 2, 885 (2012).
Am Busch, M. S., Müh, F., Madjet, M. E. & Renger, T. The eighth bacteriochlorophyll completes the excitation energy funnel in the FMO protein. J. Phys. Chem. Lett. 2, 93–98 (2011).
Olbrich, C. et al. From atomistic modeling to excitation transfer and two-dimensional spectra of the FMO light-harvesting complex. J. Phys. Chem. B 115, 8609–8621 (2011).
Ringsmuth, A. K., Milburn, G. J. & Stace, T. M. Multiscale photosynthetic and biomimetic excitation energy transfer. Nature Phys. 8, 562–567 (2012).
Dostál, J. et al. Two-dimensional electronic spectroscopy reveals ultrafast energy diffusion in chlorosomes. J. Am. Chem. Soc. 134, 11611–11617 (2012).
Hoyer, S., Sarovar, M. & Whaley, K. B. Limits of quantum speedup in photosynthetic light harvesting. New J. Phys. 12, 065041 (2010).
Cheng, Y-C. & Silbey, R. J. Coherence in the B800 ring of purple bacteria LH2. Phys. Rev. Lett. 96, 028103 (2006).
Caycedo-Soler, F., Rodriguez, F. J., Quiroga, L. & Johnson, N. F. Light-harvesting mechanism of bacteria exploits a critical interplay between the dynamics of transport and trapping. Phys. Rev. Lett. 104, 158302 (2010).
Horton, P., Johnson, M. P., Perez-Bueno, M. L., Kiss, A. Z. & Ruban, A. V. Photosynthetic acclimation: Does the dynamic structure and macro-organisation of photosystem II in higher plant grana membranes regulate light harvesting states? FEBS J. 275, 1069–1079 (2008).
Wiltschko, R., Stapput, K., Thalau, P. & Wiltschko, W. Directional orientation of birds by the magnetic field under different light conditions. J. R. Soc. Interf. 7, 163–177 (2010).
Treiber, C. D. et al. Clusters of iron-rich cells in the upper beak of pigeons are macrophages not magnetosensitive neurons. Nature 484, 367–370 (2012).
Wiltschko, W. & Wiltschko, R. Magnetic compass of european robins. Science 176, 62–64 (1972).
Wiltschko, W., Wiltschko, R. & Munro, U. Light-dependent magnetoreception in birds: Does directional information change with light intensity? Naturwissenschaften 87, 36–40 (2000).
Wiltschko, W., Stapput, K., Thalau, P. & Wiltschko, R. Avian magnetic compass: Fast adjustment to intensities outside the normal functional window. Naturwissenschaften 93, 300–304 (2006).
Ritz, T. Quantum effects in biology: Bird navigation. Proc. Chem. 3, 262–275 (2011).
Wiltschko, W., Traudt, J., Güntürkün, O., Prior, H. & Wiltschko, R. Lateralization of magnetic compass orientation in a migratory bird. Nature 419, 467–470 (2002).
Ritz, T., Thalau, P., Phillips, J. B., Wiltschko, R. & Wiltschko, W. Resonance effects indicate a radical pair mechanism for avian magnetic compass. Nature 429, 177–180 (2004).
Schulten, K., Swenberg, C. E. & Weller, A. A biomagnetic sensory mechanism based on magnetic field modulated coherent electron spin motion. Z. Phys. Chem. 111, 1–5 (1978).
Ritz, T., Adem, S. & Schulten, K. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 78, 707–718 (2000).
Rodgers, C. T. & Hore, P. J. Chemical magnetoreception in birds: The radical pair mechanism. Proc. Natl Acad. Sci. USA 106, 353–360 (2009).
Ritz, T., Ahmad, M., Mouritsen, H., Wiltschko, R. & Wiltschko, W. Photoreceptor-based magnetoreception: Optimal design of receptor molecules, cells, and neuronal processing. J. R. Soc. Interf. 7, S135–S146 (2010).
Gauger, E. M., Rieper, E., Morton, J. J. L., Benjamin, S. C. & Vedral, V. Sustained quantum coherence and entanglement in the avian compass. Phys. Rev. Lett. 106, 040503 (2011).
Steiner, U. & Ulrich, T. Magnetic field effects in chemical kinetics and related phenomena. Chem. Rev. 89, 51–147 (1989).
Woodward, J. R., Timmel, C. R., McLauchlan, K. A. & Hore, P. J. Radio frequency magnetic field effects on electron–hole recombination. Phys. Rev. Lett. 87, 077602 (2001).
Maeda, K. et al. Chemical compass model of avian magnetoreception. Nature 453, 387–390 (2008).
Cai, J., Caruso, F. & Plenio, M. B. Quantum limits for the magnetic sensitivity of a chemical compass. Phys. Rev. A 85, 040304(R) (2012).
Stoneham, A. M., Gauger, E. M., Porfyrakis, K., Benjamin, S. C. & Lovett, B. W. A new type of radical-pair-based model for magnetoreception. Biophys. J. 102, 961–968 (2012).
Wu, L-Q. & Dickman, J. D. Neural correlates of a magnetic sense. Science 336, 1054–1057 (2012).
Wiltschko, W. & Wiltschko, R. Magnetic orientation and magnetoreception in birds and other animals. J. Comp. Physiol. A 191, 675–693 (2005).
Ritz, T. et al. Magnetic compass of birds is based on a molecule with optimal directional sensitivity. Biophys. J. 96, 3451–3457 (2009).
Bandyopadhyay, J. N., Paterek, T. & Kaszlikowski, D. Quantum coherence and sensitivity of avian magnetoreception. Phys. Rev. Lett. 109, 110502 (2012).
Cai, J., Guerreschi, G. G. & Briegel, H. J. Quantum control and entanglement in a chemical compass. Phys. Rev. Lett. 104, 220502 (2010).
Gray, H. B. & Winkler, J. R. Electron tunneling through proteins. Quat. Rev. Biophys. 36, 341–372 (2003).
Stuchebrukhov, A. A. Long-distance electron tunneling in proteins. Theor. Chem. Acc. 110, 291–306 (2003).
Gray, H. B. & Winkler, J. R. Long-range electron transfer. Proc. Natl Acad. Sci. USA 102, 3534–3539 (2005).
Nagel, Z. D. & Klinman, J. P. Tunneling and dynamics in enzymatic hydride transfer. Chem. Rev. 106, 3095–3118 (2006).
Allemann, R. K. & Scrutton, N. S. Quantum Tunnelling in Enzyme-catalysed Reactions (RSC, 2009).
Stuchebrukhov, A. A. Long-distance electron tunneling in proteins: A new challenge for time-resolved spectroscopy. Laser Phys. 20, 125–138 (2010).
Moser, C. C., Anderson, J. L. R. & Dutton, P. L. Guidelines for tunneling in enzymes. BBA—Bioenergetics 1797, 1573–1586 (2010).
Regan, J. & Onuchic, J. N. Electron-transfer tubes. Adv. Chem. Phys. 107, 497–553 (1999).
Onuchic, J., Beratan, D., Winkler, J. & Gray, H. Pathway analysis of protein electron-transfer reactions. Ann. Rev. Biophys. Biomol. Struct. 21, 349–369 (1992).
Xiao, D., Skourtis, S. S., Rubtsov, I. V. & Beratan, D. N. Turning charge transfer on and off in a molecular interferometer with vibronic pathways. Nano Lett. 9, 1818–1823 (2009).
Skourtis, S. S., Waldeck, D. H. & Beratan, D. N. Fluctuations in biological and bioinspired electron-transfer reactions. Annu. Rev. Phys. Chem. 61, 461–485 (2010).
Turin, L. A spectroscopic mechanism for primary olfactory reception. Chem. Senses 21, 773–791 (1996).
Brookes, J. C., Hartoutsiou, F., Horsfield, A. P. & Stoneham, A. M. Could humans recognize odor by phonon-assisted tunneling? Phys. Rev. Lett. 98, 038101 (2007).
Van der Horst, M. A. & Hellingwerf, K. J. Photoreceptor proteins, ‘star actors of modern times’: A review of the functional dynamics in the structure of representative members of six different photoreceptor families. Acc. Chem. Res. 37, 13–20 (2004).
Sundstrom, V. Femtobiology. Annu. Rev. Phys. Chem. 59, 53–77 (2008).
Onuchic, J. & Wolynes, P. Classical and quantum pictures of reaction dynamics in condensed matter—resonances, dephasing, and all that. J. Phys. Chem. 92, 6495–6503 (1988).
Schoenlein, R. W., Peteanu, L., Mathies, R. A. & Shank, C. The first step in vision: Femtosecond isomerization of rhodopsin. Science 254, 412–415 (1991).
Abe, M., Ohtsuki, Y., Fujimura, Y. & Domcke, W. Optimal control of ultrafast cis-trans photoisomerization of retinal in rhodopsin via a conical intersection. J. Chem. Phys. 123, 144508–144508 (2005).
Polli, D. et al. Conical intersection dynamics of the primary photoisomerization event in vision. Nature 467, 440–443 (2010).
Ben-Nun, M. et al. Quantum dynamics of the femtosecond photoisomerization of retinal in bacteriorhodopsin. Faraday Discuss. 110, 447–462 (1998).
Prokhorenko, V. I. et al. Coherent control of retinal isomerization in bacteriorhodopsin. Science 313, 1257–1261 (2006).
Prokhorenko, V. I., Halpin, A., Johnson, P. J. M., Miller, R. J. D. & Brown, L. S. Coherent control of the isomerization of retinal in bacteriorhodopsin in the high intensity regime. J. Chem. Phys. 134, 085105 (2011).
Sarovar, M., Ishizaki, A., Fleming, G. R. & Whaley, K. B. Quantum entanglement in photosynthetic light-harvesting complexes. Nature Phys. 6, 462–467 (2010).
Wiltschko, W. et al. The magnetic compass of domestic chickens, Gallus gallus. J. Exp. Biol. 210, 2300–2310 (2007).
Rodgers, C. T., Norman, S. A., Henbest, K. B., Timmel, C. R. & Hore, P. J. Determination of radical re-encounter probability distributions from magnetic field effects on reaction yields. J. Am. Chem. Soc. 129, 6746–6755 (2007).
Acknowledgements
We thank A. Pisliakov, L. Valkunas, E. Gauger, P. Nation, S. Darroch, I. Mahboob, A. Y. Smirnov, A. Ishizaki, K. Jacobs and S. De Liberato for helpful discussions and feedback. Y-C.C. thanks the National Science Council, Taiwan (Grant No. NSC 100-2113-M-002-004-MY2), the National Taiwan University (Grant No. 10R80912-5) and the Center for Quantum Science and Engineering (Subproject: 10R80914-1) for financial support. Y-N.C. thanks the National Science Council, Taiwan (Grant No. NSC 101-2628-M-006-003-MY3) for financial support. F.N. acknowledges partial support from the ARO, JSPS-RFBR contract No. 12-02-92100, MEXT Kakenhi on Quantum Cybernetics and the JSPS-FIRST Program. C-M.L thanks the National Science Council, Taiwan (No. NSC 101-2112-M-006-016-MY3, No. NSC 101-2738-M-006-005 and No. NSC 103-2911-I-006 -301) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lambert, N., Chen, YN., Cheng, YC. et al. Quantum biology. Nature Phys 9, 10–18 (2013). https://doi.org/10.1038/nphys2474
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphys2474
This article is cited by
-
Wireless electrical–molecular quantum signalling for cancer cell apoptosis
Nature Nanotechnology (2024)
-
Essential role of momentum-forbidden dark excitons in the energy transfer responses of monolayer transition-metal dichalcogenides
npj 2D Materials and Applications (2023)
-
Energy transfer in N-component nanosystems enhanced by pulse-driven vibronic many-body entanglement
Scientific Reports (2023)
-
Hot entanglement? — Parametrically coupled quantum oscillators in two heat baths: instability, squeezing and driving
Journal of High Energy Physics (2023)
-
Quantum algorithm of Dempster rule of combination
Applied Intelligence (2023)