Abstract
Hydrogen, being the first element in the periodic table, has the simplest electronic structure of any atom, and the hydrogen molecule contains the simplest covalent chemical bond. Nevertheless, the phase diagram of hydrogen is poorly understood. Determining the stable structures of solid hydrogen is a tremendous experimental challenge1,2,3, because hydrogen atoms scatter X-rays only weakly, leading to low-resolution diffraction patterns. Theoretical studies encounter major difficulties owing to the small energy differences between structures and the importance of the zero-point motion of the protons. We have systematically investigated the zero-temperature phase diagram of solid hydrogen using first-principles density functional theory (DFT) electronic-structure methods4, including the proton zero-point motion at the harmonic level. Our study leads to a radical revision of the DFT phase diagram of hydrogen up to nearly 400 GPa. That the most stable phases remain insulating to very high pressures eliminates a major discrepancy between theory5 and experiment6. One of our new phases is calculated to be stable over a wide range of pressures, and its vibrational properties agree with the available experimental data for phase III.
Similar content being viewed by others
Main
The low-pressure phase I of solid hydrogen, which consists of freely rotating molecules on a hexagonal close-packed lattice2, transforms at pressures of about 110 GPa to the broken-symmetry phase II, in which the mean molecular orientations are ordered, and then to phase III at about 150 GPa (ref. 1). However, even the combination of X-ray and neutron scattering data and Raman and infrared vibrational data has not so far yielded the structures of phases II and III of hydrogen.
The theoretical prediction of stable crystal structures is very difficult because of the need to search the very large space of possible structures, and the necessity of obtaining accurate energies for each of these structures. First-principles DFT methods have proved an efficient means of calculating quite accurate energies, and they have provided many insights into the properties of materials, including solid hydrogen5,7. At present, DFT offers the highest level of theoretical description at which we can carry out searches over many possible candidate structures.
Our approach is to relax many random structures to minima in the enthalpy at fixed pressure8. This method does not rely on previous theoretical or experimental results, and it allows for the possibility of finding radically new structures. In some cases we used the intuition gained from the random searches to build other candidate structures. We then calculated the enthalpies of the most stable phases at a larger number of pressures, generating the data shown in Fig. 1. We refer to each structure by its short Hermann–Mauguin space-group symbol, giving additional information where an ambiguity might occur.
The lowest-enthalpy structures found around 100 GPa were those of space groups P c a21 and P21/c, which were considered in previous studies5,7, and a new structure of space group P63/m, which is marginally the most stable up to 105 GPa. The centres of the molecules in these structures form a slightly distorted hexagonal close-packed lattice, as is generally thought to occur in phase II of solid hydrogen. The molecular bond lengths of the different structures are very similar and the molecules are orientated so that the atoms of neighbouring molecules are kept apart. The P63/m structure differs from the other two in that some molecules lie perpendicular to the plane, whereas the others are arranged around it in a ‘swirl’; see Fig. 2.
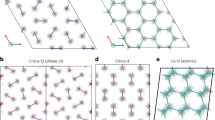
The layers are stacked in an ABAB fashion, and the primitive unit cell contains 16 atoms, which form two types of hydrogen molecule. Three-quarters of the molecules lie flat within the plane, and one-quarter lie perpendicular to the plane. The centres of the molecules lie on a slightly distorted hexagonal close-packed lattice. In this and Figs 3 and 4, bonds are indicated between atoms closer than 1.05 Å, and close contacts (pink dashed lines), if they exist, between atoms closer than 1.15 Å.
The most stable phase we found in the range 105–270 GPa is a layered structure of space group C2/c, which is illustrated in Fig. 3. We also found a layered molecular structure of Cmca symmetry with a 12-atom primitive unit cell. We refer to this as Cmca-12 to distinguish it from the Cmca structure discussed in earlier work5, which was also generated by our searches. Cmca-12 is illustrated in Fig. 4, and it is the most stable phase in the range 270–385 GPa. From 385–490 GPa we found the Cmca structure to be the most stable, and above 490 GPa the monatomic I41/a m d structure with a c/a ratio greater than unity (which is also the structure of Cs-IV) is most stable. Cmca-12 remains competitive up to about 500 GPa, and a more closely packed version of Cmca, which we refer to as high-Cmca (and is the same structure as black phosphorus), is almost degenerate with Cmca-12 and I41/a m d at about 480 GPa. A structure of C m c m symmetry has been obtained by following an unstable phonon displacement of the P c a21 structure9. We have studied this structure but found it to be uncompetitive; see Fig. 1. We found numerous less stable structures, including a particularly intriguing family of ‘mixed’ structures consisting of alternate layers of strongly bonded hydrogen molecules and less strongly bonded atoms, which at higher pressures resemble graphene layers. These mixed structures are described in more detail in the Supplementary Information.
The layers are arranged in an ABCDA fashion, and the primitive unit cell contains 24 atoms. Each layer consists of three inequivalent molecules whose axes are nearly parallel to the plane and whose centres form a distorted hexagonal lattice. Note the asymmetry between the two ends of the molecules, which leads to the strong infrared activity in this structure.
The small mass of the proton poses a significant problem for theoretical descriptions of hydrogen; the zero-point (ZP) motion of the nuclei is large enough to significantly affect the relative stabilities of structures and their vibrational properties. We have estimated ZP vibrational energies within DFT using the harmonic approximation. The harmonic approximation is likely to give useful estimates of the total ZP vibrational energy of the candidate structures at low pressures, as the calculated harmonic vibronic frequencies are similar to the measured values. There are, however, noticeable anharmonic effects in hydrogen, even at low pressures1,10. In monatomic structures, which are believed to be stable at very high pressures, the harmonic approximation fails completely11.
Our calculated ZP energy of the free hydrogen molecule of 0.133 eV per proton is a little larger than the experimental value of 0.129 eV per proton12, the majority of the error arising from the harmonic approximation. The calculated ZP energies at 75 GPa are roughly 0.25 eV per proton, rising to about 0.28 eV per proton at 150 GPa. The ZP pressures are quite small, being roughly 6 GPa at a static lattice pressure of 75 GPa, rising to roughly 8 GPa at 150 GPa. The ZP enthalpies are large on the scale of Fig. 1. There is a strong tendency for them to cancel between the different phases, but the resulting changes in their relative stabilities are significant. Comparing the main and inset graphs of Fig. 1, we see that ZP effects favour the C2/c and Cmca phases and the mixed C2 and Pbcn phases over the P21/c, P c a21 and P63/m phases. The predicted range of stability of C2/c then extends to lower pressures. The ZP effects reduce the upper pressure at which C2/c is stable to 240 GPa, where it yields to Cmca-12, although Pbcn is very close in enthalpy at this pressure.
Our results lead to a complete revision of the DFT phase diagram of hydrogen. Within the static-lattice approximation, the most stable phases are P63/m (<105 GPa), C2/c (105–270 GPa), Cmca-12 (270–385 GPa) and Cmca (385–490 GPa), followed by I41/a m d. Of these phases, only Cmca and I41/a m d were known previously. When ZP effects are included, C2/c becomes stable below 240 GPa and Cmca-12 above. The enthalpies of some of the phases are very similar, and small changes in their relative enthalpies could significantly alter the stability regions of the different phases.
The calculated densities of electronic states show that Cmca-12 becomes metallic at about 240 GPa, C2 at about 260 GPa and the C2/c and Pbcn phases at about 300 GPa. Given that standard DFT approaches normally underestimate bandgaps, the true metallization pressures are likely to be significantly higher. Exact-exchange DFT calculations indicate that the true bandgaps in hydrogen solids are roughly 1–2 eV higher than the standard DFT values13. Increasing our calculated gaps by 1 eV and extrapolating to the pressure at which the gap vanishes gives metallization pressures of about 350 GPa for Pbcn and C2/c, whereas an increase of 2 eV gives metallization at about 410 GPa. These pressures are consistent with the experimental finding that hydrogen remains insulating up to at least 320 GPa (ref. 6).
We have calculated the infrared intensities of the vibrational modes and identified the Raman-active vibron modes of the most significant structures; see Fig. 5 and the Supplementary Information. Phase II seems to have three infrared-active vibron modes, but overall its infrared activity is low1. P c a21 has three infrared-active vibron modes and a low overall infrared activity, whereas P63/m has only one infrared-active vibron and a significantly larger overall infrared activity. P63/m is therefore inconsistent with the infrared data.
Only the most strongly infrared-active modes are shown; the intensities of all other infrared-active modes are at least an order of magnitude smaller. The calculated infrared frequencies are shown as red solid lines, with the line width being proportional to the intensity. The Raman-active vibron mode is shown as a blue solid line. Experimental data from ref. 19 are shown as dashed lines.
The structures formed by the heavier isotope deuterium are expected to be similar to those of hydrogen. Recent experiments3 indicate that phase II of deuterium has an incommensurate structure, although a periodic model of  symmetry was suggested as having the same local ordering of molecules. We found this molecular structure to be poorly packed, and relaxation with the constraint that the symmetry is not lowered led to a structure of higher P63/m m c symmetry, which, however, is high in enthalpy, as shown in Fig. 1. We therefore conclude that the even less stable
symmetry was suggested as having the same local ordering of molecules. We found this molecular structure to be poorly packed, and relaxation with the constraint that the symmetry is not lowered led to a structure of higher P63/m m c symmetry, which, however, is high in enthalpy, as shown in Fig. 1. We therefore conclude that the even less stable  structure is unlikely to be related to phase II. (A similar conclusion was arrived at in ref. 14.) On the other hand, the neutron data for deuterium of ref. 3 were found to be incompatible with the P c a21 and P21/c structures.
structure is unlikely to be related to phase II. (A similar conclusion was arrived at in ref. 14.) On the other hand, the neutron data for deuterium of ref. 3 were found to be incompatible with the P c a21 and P21/c structures.
The very small volume change and apparently continuous behaviour of the Raman vibron frequency across the I–II transition suggests that phase II may consist of an arrangement of molecules on a hexagonal, or nearly hexagonal, close-packed lattice. Although the P21/c, P c a21 and P63/m structures are of this type, it is possible that none of the structures suggested so far is compatible with phase II of hydrogen or deuterium, and the level of theory used may be inadequate. For example, our calculations do not account for the differences between ortho and para molecules, which plays an important role in phases I and II of hydrogen and deuterium15. Although the mean molecular orientations are ordered in phase II, very wide-angle librational motion occurs, and the phase coherence between the orientations of molecules is weak. Our DFT calculations show that a number of packings of molecules (all on the basis of a slightly distorted hexagonal close-packed lattice) have very similar enthalpies in the pressure range appropriate for phase II, suggesting that several significantly different local molecular configurations might contribute to the overall structure. A full understanding of phase II presents a difficult theoretical challenge.
The distinction between ortho and para molecules is believed to be unimportant in phase III because the molecules have much less freedom to rotate in this compressed structure. Two of the most notable characteristics of phase III are its much greater infrared activity than phase II and the appearance of a single intense infrared active vibron peak16. The Cmca-12 structure shows two fairly weakly infrared-active vibron modes separated by between 200 and 250 cm−1. The overall infrared intensity of Cmca-12 is calculated to be just over twice that in P c a21 and is actually less than that in P63/m, and it seems likely that the infrared intensity of Cmca-12 is too small to be consistent with phase III. The Cmca phase, found to be stable over a very large pressure range in previous DFT studies5, is ruled out as a candidate for phase III because it is metallic and its vibrons are much softer than the observed ones, and we have found considerably more stable phases over most of the relevant pressure range.
Our new C2/c structure is a strong candidate for phase III. C2/c shows intense infrared vibron activity arising from the increased charge asymmetry between the two ends of the hydrogen molecules; see Fig. 3. Our calculations show an increase in overall infrared activity in C2/c over that in P c a21 by a factor of six. The increase for the vibron modes is much larger, the infrared intensity of the vibrons in C2/c being about 150 times larger than in P c a21. C2/c has six infrared-active vibrons, three of which are strong and differ by less than 25 cm−1 over a very wide range of pressures (see Fig. 5) and would therefore appear as a single peak in the infrared absorption spectrum, in agreement with experiment. The two strongest differ by less than 15 cm−1, and account for 95% of the intensity of the combined peak. The intensities of the other three infrared-active vibrons are each less than 1% of the intensity of the strong infrared vibron peak. The infrared activity of the strong infrared-active vibrons in C2/c increases with pressure, as is observed in phase III17. Figure 5 shows the variation with pressure of both the strong infrared peak and the Raman-active vibron frequency of C2/c to be in good agreement with experiment18. Figure 5 also shows a fairly strongly infrared-active phonon mode between 1,600 and 2,000 cm−1, as seen in experiment18. The Supplementary Information shows that the calculated libron frequencies (those up to about 600 cm−1) appear in the correct frequency range and gently increase with pressure in a similar fashion to those observed in experiment18.
Methods
We used the CASTEP plane-wave code19, the Perdew–Burke–Ernzerhof generalized gradient approximation density functional20 and ultrasoft pseudopotentials21. We carried out very extensive random searches at pressures from 100 to 600 GPa in steps of 100 GPa, using unit cells containing eight and 12 atoms, and other searches using up to 48-atom cells at some pressures. We calculated the phonon frequencies for the ZP energies using a finite-difference method and the infrared intensities using density functional perturbation theory22; see the Supplementary Information for more details.
References
Mao, H. K. & Hemley, R. J. Ultrahigh-pressure transitions in solid hydrogen. Rev. Mod. Phys. 66, 671–692 (1994).
Loubeyre, P. et al. X-ray diffraction and equation of state of hydrogen at megabar pressures. Nature 383, 702–704 (1996).
Goncharenko, I. & Loubeyre, P. Neutron and X-ray diffraction study of the broken symmetry phase transition in solid deuterium. Nature 435, 1206–1209 (2005).
Martin, R. M. Electronic Structure: Basic Theory and Practical Methods (Cambridge Univ. Press, Cambridge, 2004).
Johnson, K. A. & Ashcroft, N. W. Structure and bandgap closure in dense hydrogen. Nature 403, 632–635 (2000).
Loubeyre, P., Occelli, F. & LeToullec, R. Optical studies of solid hydrogen to 320 GPa and evidence for black hydrogen. Nature 416, 613–617 (2002).
Kohanoff, J., Scandolo, S., Chiarotti, G. L. & Tosatti, E. Solid molecular hydrogen: The broken symmetry phase. Phys. Rev. Lett. 78, 2783–2786 (1997).
Pickard, C. J. & Needs, R. J. High pressure phases of silane. Phys. Rev. Lett. 97, 045504 (2006).
Kohanoff, J., Scandolo, S., de Gironcoli, S. & Tosatti, E. Dipole–quadrupole interactions and the nature of Phase III of compressed hydrogen. Phys. Rev. Lett. 83, 4097 (1999).
Ashcroft, N. W. Pairing instabilities in dense hydrogen. Phys. Rev. B 41, 10963–10971 (1990).
Natoli, V., Martin, R. M. & Ceperley, D. M. Crystal structure of atomic hydrogen. Phys. Rev. Lett. 70, 1952–1955 (1993).
Dabrowski, I. The Lyman and Werner bands of H2 . Can. J. Phys. 62, 1639–1664 (1984).
Städele, M. & Martin, R. M. Metallization of molecular hydrogen: Predictions from exact-exchange calculations. Phys. Rev. Lett. 84, 6070–6073 (2000).
Zhang, L. J. et al. Ab initio lattice dynamics evidence for the broken-symmetry phase of solid hydrogen. J. Phys. Condens. Matter 18, 9917–9927 (2006).
Goncharov, A. F., Eggert, J. H., Mazin, I. I., Hemley, R. J. & Mao, H. K. Raman excitations and orientational ordering in deuterium at high pressure. Phys. Rev. B 54, 15590–15593 (1996).
Hemley, R. J., Soos, Z. G., Hanfland, M. & Mao, H.-K. Charge-transfer states in dense hydrogen. Nature 369, 384–387 (1994).
Hemley, R. J., Mazin, I. I., Goncharov, A. F. & Mao, H. K. Vibron effective charges in dense hydrogen. Europhys. Lett. 37, 403–407 (1997).
Goncharov, A. F., Gregoryanz, E., Hemley, R. J. & Mao, H.-K. Spectroscopic studies of the vibrational and electronic properties of solid hydrogen to 285 GPa. Proc. Natl Acad. Sci. USA 98, 14234–14237 (2001).
Clark, S. J. et al. First principles methods using CASTEP. Z. Kristallogr. 220, 567–570 (2005).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 41, 7892–7895 (1990).
Refson, K., Tulip, P. R. & Clark, S. J. Variational density functional perturbation theory for dielectrics and lattice dynamics. Phys. Rev. B 73, 155114 (2006).
Acknowledgements
C.J.P. was supported by an EPSRC Advanced Research Fellowship. We thank K. Refson for assistance in carrying out the phonon calculations and J. Kohanoff for useful discussions, including the information that he and G. Grad had also studied the Cmca-12 phase.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
C.J.P. Is an author of the castep code, which is marketed commercially by Accelrys. He receives royalties on the sales.
Supplementary information
Rights and permissions
About this article
Cite this article
Pickard, C., Needs, R. Structure of phase III of solid hydrogen. Nature Phys 3, 473–476 (2007). https://doi.org/10.1038/nphys625
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphys625
This article is cited by
-
Small-pore hydridic frameworks store densely packed hydrogen
Nature Chemistry (2024)
-
Quantum structural fluxion in superconducting lanthanum polyhydride
Nature Communications (2023)
-
Quantum phase diagram of high-pressure hydrogen
Nature Physics (2023)
-
Enhanced superconducting transition temperature via alloying In, Sn and Sb in PbH4 by using first-principles calculations
Journal of Materials Science (2023)
-
Stabilizing superconductivity of ternary metal pentahydride \(\hbox {CaCH}_{{5}}\) via electronic topological transitions under high pressure from first principles evolutionary algorithm
Scientific Reports (2022)