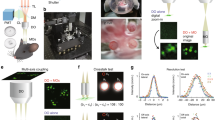
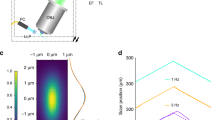
Abstract
We report a three-dimensional microscopy technique—swept, confocally-aligned planar excitation (SCAPE) microscopy—that allows volumetric imaging of living samples at ultrahigh speeds. Although confocal and two-photon microscopy have revolutionized biomedical research, current implementations are costly, complex and limited in their ability to image three-dimensional volumes at high speeds. Light-sheet microscopy techniques using two-objective, orthogonal illumination and detection require a highly constrained sample geometry and either physical sample translation or complex synchronization of illumination and detection planes. In contrast, SCAPE microscopy acquires images using an angled, swept light sheet in a single-objective, en face geometry. Unique confocal descanning and image rotation optics map this moving plane onto a stationary high-speed camera, permitting completely translationless three-dimensional imaging of intact samples at rates exceeding 20 volumes per second. We demonstrate SCAPE microscopy by imaging spontaneous neuronal firing in the intact brain of awake behaving mice, as well as freely moving transgenic Drosophila larvae.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Akerboom, J. et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci. 32, 13819–13840 (2012).
Chen, T.-W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Dodt, H.-U. et al. Ultramicroscopy: three-dimensional visualization of neuronal networks in the whole mouse brain. Nature Methods 4, 331–336 (2007).
Verveer, P. J. et al. High-resolution three-dimensional imaging of large specimens with light sheet-based microscopy. Nature Methods 4, 311–313 (2007).
Keller, P. J. et al. Fast, high-contrast imaging of animal development with scanned light sheet-based structured-illumination microscopy. Nature Methods 7, 637–642 (2010).
Ahrens, M. B., Orger, M. B., Robson, D. N., Li, J. M. & Keller, P. J. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nature Methods 10, 413–420 (2013).
Fahrbach, F. O., Voigt, F. F., Schmid, B., Helmchen, F. & Huisken, J. Rapid 3D light-sheet microscopy with a tunable lens. Opt. Express 21, 21010–21026 (2013).
Holekamp, T. F., Turaga, D. & Holy, T. E. Fast three-dimensional fluorescence imaging of activity in neural populations by objective-coupled planar illumination microscopy. Neuron 57, 661–672 (2008).
Wu, Y. et al. Inverted selective plane illumination microscopy (iSPIM) enables coupled cell identity lineaging and neurodevelopmental imaging in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 108, 17708–17713 (2011).
Kumar, S. et al. High-speed 2D and 3D fluorescence microscopy of cardiac myocytes. Opt. Express 19, 13839–13847 (2011).
Kumar, S. et al. High-speed 2D and 3D fluorescence microscopy of cardiac myocytes. Opt. Express 19, 13839–13847 (2011).
Gobel, W., Kampa, B. M. & Helmchen, F. Imaging cellular network dynamics in three dimensions using fast 3D laser scanning. Nature Methods 4, 73–79 (2007).
Glickfeld, L. L., Andermann, M. L., Bonin, V. & Reid, R. C. Cortico-cortical projections in mouse visual cortex are functionally target specific. Nature Neurosci. 16, 219–226 (2013).
Jia, H., Varga, Z., Sakmann, B. & Konnerth, A. Linear integration of spine Ca2+ signals in layer 4 cortical neurons in vivo. Proc. Natl Acad. Sci. USA 111, 9277–9282 (2014).
Mittmann, W. et al. Two-photon calcium imaging of evoked activity from L5 somatosensory neurons in vivo. Nature Neurosci. 14,1089–1093 (2011).
Schrodel, T., Prevedel, R., Aumayr, K., Zimmer, M. & Vaziri, A. Brain-wide 3D imaging of neuronal activity in Caenorhabditis elegans with sculpted light. Nature Methods 10, 1013–1020 (2013).
Katona, G. et al. Fast two-photon in vivo imaging with three-dimensional random-access scanning in large tissue volumes. Nature Methods 9, 201–208 (2012).
Grewe, B. F., Langer, D., Kasper, H., Kampa, B. M. & Helmchen, F. High-speed in vivo calcium imaging reveals neuronal network activity with near-millisecond precision. Nature Methods 7, 399–405 (2010).
Cotton, R. J., Froudarakis, E., Storer, P., Saggau, P. & Tolias, A. S. Three-dimensional mapping of microcircuit correlation structure. Front. Neural Circ. 7, 151 (2013).
Dwyer, P. J., DiMarzio, C. A., Zavislan, J. M., Fox, W. J. & Rajadhyaksha, M. Confocal reflectance theta line scanning microscope for imaging human skin in vivo. Opt. Lett. 31, 942–944 (2006).
Dunsby, C. Optically sectioned imaging by oblique plane microscopy. Opt. Express 16, 20306–20316 (2008).
Vaziri, A. & Shank, C. V. Ultrafast widefield optical sectioning microscopy by multifocal temporal focusing. Opt. Express 18, 19645–19655 (2010).
Hillman, E. M. C. & Moore, A. All-optical anatomical co-registration for molecular imaging of small animals using dynamic contrast. Nature Photon. 1, 526–530 (2007).
Schuster, C. M., Davis, G. W., Fetter, R. D. & Goodman, C. S. Genetic dissection of structural and functional components of synaptic plasticity. I. Fasciclin II controls synaptic stabilization and growth. Neuron 17, 641–654 (1996).
Curtis, N. J., Ringo, J. M. & Dowse, H. B. Morphology of the pupal heart, adult heart, and associated tissues in the fruit fly, Drosophila melanogaster. J. Morphol. 240, 225–235 (1999).
Bouchard, M. B. et al. Technical considerations in longitudinal multispectral small animal molecular imaging. J. Biomed. Opt. 12, 051601 (2007).
Broxton, M. et al. Wave optics theory and 3-D deconvolution for the light field microscope. Opt. Express 21, 25418–25439 (2013).
Quirin, S., Jackson, J., Peterka, D. S. & Yuste, R. Simultaneous imaging of neural activity in three dimensions. Front. Neural Circ. 8, 29 (2014).
Akerboom, J. et al. Genetically encoded calcium indicators for multi-color neural activity imaging and combination with optogenetics. Front. Mol. Neurosci. 6, 2 (2013).
Hillman, E. M. C., Boas, D. A., Dale, A. M. & Dunn, A. K. Laminar optical tomography: demonstration of millimeter-scale depth-resolved imaging in turbid media. Opt. Lett. 29, 1650–1652 (2004).
Truong, T. V., Supatto, W., Koos, D. S., Choi, J. M. & Fraser, S. E. Deep and fast live imaging with two-photon scanned light-sheet microscopy. Nature Methods 8, 757–760 (2011).
Horton, N. G. et al. In vivo three-photon microscopy of subcortical structures within an intact mouse brain. Nature Photon. 7, 205–209 (2013).
Kobat, D. et al. Deep tissue multiphoton microscopy using longer wavelength excitation. Opt. Express 17, 13354–13364 (2009).
Lavagnino, Z., Zanacchi, F. C., Ronzitti, E. & Diaspro, A. Two-photon excitation selective plane illumination microscopy (2PE-SPIM) of highly scattering samples: characterization and application. Opt. Express 21, 5998–6008 (2013).
Friedrich, M., Gan, Q., Ermolayev, V. & Harms, G. S. STED-SPIM stimulated emission depletion improves sheet illumination microscopy resolution. Biophys. J. 100, L43–L45 (2011).
Planchon, T. A. et al. Rapid three-dimensional isotropic imaging of living cells using Bessel beam plane illumination. Nature Methods 8, 417–423 (2011).
Lutz, C. et al. Holographic photolysis of caged neurotransmitters. Nature Methods 5, 821–827 (2008).
Golan, L., Reutsky, I., Farah, N. & Shoham, S. Design and characteristics of holographic neural photo-stimulation systems. J. Neural Eng. 6, 066004 (2009).
Jing, D. et al. In situ intracellular calcium oscillations in osteocytes in intact mouse long bones under dynamic mechanical loading. FASEB J. 28, 1582–1592 (2014).
Carlson, G. C. & Coulter, D. A. In vitro functional imaging in brain slices using fast voltage-sensitive dye imaging combined with whole-cell patch recording. Nature Protoc. 3, 249–255 (2008).
Xie, W. et al. Imaging atrial arrhythmic intracellular calcium in intact heart. J. Mol. Cell. Cardiol. 64, 120–123 (2013).
Sung, Y. et al. Three-dimensional holographic refractive-index measurement of continuously flowing cells in a microfluidic channel. Phys. Rev. Appl. 1, 014002 (2014).
Regmi, R., Mohan, K. & Mondal, P. P. Light sheet based imaging flow cytometry on a microfluidic platform. Microsc. Res. Tech. 76, 1101–1107 (2013).
Baik, A. D., Qiu, J., Hillman, E. M. C., Dong, C. & Guo, X. E. Simultaneous tracking of 3D actin and microtubule strains in individual MLO-Y4 osteocytes under oscillatory flow. Biochem. Biophys. Res. Commun. 431, 718–723 (2013).
Radosevich, A. J., Bouchard, M. B., Burgess, S. A., Chen, B. R. & Hillman, E. M. C. Hyperspectral in vivo two-photon microscopy of intrinsic contrast. Opt. Lett. 33, 2164–2166 (2008).
Acknowledgements
The authors acknowledge the contributions of R. Yuste, D. Kelley and M. Chalfie and their students and staff (in particular M. Agetsuma, S. Quirin and D. Peterka) for assistance with early sample selection and preparation, as well as the Bloomington Stock Center, S. Galindo, M. Baylies and B. Noro for fly stocks. K. Yeager, L.E. Grosberg, M. Shaik, M. Kozberg, S. Kim, Y. Ma, T.J. Muldoon and S. Qian provided assistance with instrumentation and sample preparation. The authors thank I. Herman and T. Galwaduge for assistance with PSF calculations, L. Paninski for discussions on neuronal data analysis and R. Levenson for guidance on applications. The authors acknowledge Lincoln Laser and Cambridge Technology for assistance with scanner fabrication. Funding was provided by NIH (NINDS) R21NS053684, R01 NS076628 and R01NS063226, NSF CAREER 0954796, The Human Frontier Science Program and the Wallace H. Coulter Foundation (E.M.C.H.), NIH (NINDS) R01 NS069679 and the Dana Foundation (R.M.B.), (NINDS) R01NS070644 (R.S.M.), (NINDS) R01NS061908 (W.B.G.), DoD MURI W911NF-12–1-0594 (Yuste). M.B. received NSF and NDSEG graduate fellowships. V.V. was funded by an NSF IGERT Fellowship. C.S.M. is supported by a postdoctoral fellowship from Fundação para a Ciência e a Tecnologia, Portugal.
Author information
Authors and Affiliations
Contributions
M.B.B. and E.M.C.H. conceived the technique. M.B.B., E.M.C.H. and V.V. generated ray-tracing models, built the system, acquired and processed data, and prepared the manuscript. C.S.M., R.S.M. and W.B.G. provided and assisted with Drosophila samples, and C.L. and R.M.B. provided and assisted with mouse models. R.S.M., C.S.M., W.B.G., C.L. and R.M.B. all advised on image interpretation and manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
A patent related to this technique was issued to The Trustees of Columbia University in the City of New York on 31 December 2013 (inventors Hillman and Bouchard).
Supplementary information
Supplementary information
Supplementary information (PDF 3830 kb)
Supplementary information
Supplementary information (PDF 579 kb)
Supplementary movie 1
Supplementary movie 1 (MOV 29435 kb)
Supplementary movie 2
Supplementary movie 2 (MOV 2447 kb)
Supplementary movie 3
Supplementary movie 3 (MOV 28204 kb)
Supplementary movie 4
Supplementary movie 4 (MOV 19884 kb)
Supplementary movie 5
Supplementary movie 5 (MOV 12484 kb)
Supplementary movie 6
Supplementary movie 6 (MOV 7745 kb)
Supplementary movie 7
Supplementary movie 7 (MOV 16905 kb)
Supplementary movie 8
Supplementary movie 8 (MOV 13566 kb)
Rights and permissions
About this article
Cite this article
Bouchard, M., Voleti, V., Mendes, C. et al. Swept confocally-aligned planar excitation (SCAPE) microscopy for high-speed volumetric imaging of behaving organisms. Nature Photon 9, 113–119 (2015). https://doi.org/10.1038/nphoton.2014.323
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphoton.2014.323
This article is cited by
-
Fluorescence-based multifunctional light sheet imaging flow cytometry for high-throughput optical interrogation of live cells
Communications Physics (2024)
-
Automated neuron tracking inside moving and deforming C. elegans using deep learning and targeted augmentation
Nature Methods (2024)
-
High-resolution open-top axially swept light sheet microscopy
BMC Biology (2023)
-
Virtual-scanning light-field microscopy for robust snapshot high-resolution volumetric imaging
Nature Methods (2023)
-
Widefield imaging of rapid pan-cortical voltage dynamics with an indicator evolved for one-photon microscopy
Nature Communications (2023)