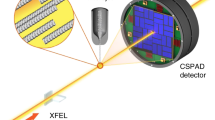
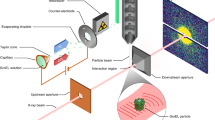
Abstract
We overcome two of the most daunting challenges in single-particle diffractive imaging: collecting many high-quality diffraction patterns on a small amount of sample and separating components from mixed samples. We demonstrate this on carboxysomes, which are polyhedral cell organelles that vary in size and facilitate up to 40% of Earth's carbon fixation. A new aerosol sample-injector allowed us to record 70,000 low-noise diffraction patterns in 12 min with the Linac Coherent Light Source running at 120 Hz. We separate different structures directly from the diffraction data and show that the size distribution is preserved during sample delivery. We automate phase retrieval and avoid reconstruction artefacts caused by missing modes. We attain the highest-resolution reconstructions on the smallest single biological objects imaged with an X-ray laser to date. These advances lay the foundations for accurate, high-throughput structure determination by flash-diffractive imaging and offer a means to study structure and structural heterogeneity in biology and elsewhere.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Neutze, R., Wouts, R., van der Spoel, D., Weckert, E. & Hajdu, J. Potential for biomolecular imaging with femtosecond X-ray pulses. Nature 406, 752–757 (2000).
Bergh, M., Huldt, G., Timneanu, N., Maia, F. R. N. C. & Hajdu, J. Feasibility of imaging living cells at subnanometer resolutions by ultrafast X-ray diffraction. Q. Rev. Biophys. 41, 181–204 (2008).
Chapman, H. N. et al. Femtosecond diffractive imaging with a soft-X-ray free-electron laser. Nature Phys. 2, 839–843 (2006).
Chapman, H. N. et al. Femtosecond X-ray protein nanocrystallography. Nature 470, 73–77 (2011).
Seibert, M. M. et al. Single mimivirus particles intercepted and imaged with an X-ray laser. Nature 470, 78–81 (2011).
Bernal, J. D., Fankuchen, I. & Perutz, M. F. An X-ray study of chymotrypsin and haemoglobin. Nature 141, 523–524 (1938).
Shannon, C. E. Communication in the presence of noise. Proc. Inst. Radio Eng. 37, 10–21 (1949).
Sayre, D. On the implication of a theorem due to Shannon. Acta Crystallogr. 5, 834 (1952).
Fienup, J. R. Reconstruction of an object from the modulus. Opt. Lett. 3, 27–29 (1978).
Gerchberg, R. W. & Saxton, W. O. A practical algorithm for the determination of the phase from image and diffraction plane pictures. Optik 35, 237–246 (1972).
Miao, J., Charalambous, P., Kirz, J. & Sayre, D. Extending the methodology of X-ray crystallography to allow imaging of micrometre-sized non-crystalline specimens. Nature 400, 342–344 (1999).
Marchesini, S. et al. X-ray image reconstruction from a diffraction pattern alone. Phys. Rev. B 68, 140101 (2003).
Luke, D. R. Relaxed averaged alternating reflections for diffraction imaging. Inverse Prob. 21, 37–50 (2005).
Huldt, G., Szöke, A. & Hajdu, J. Diffraction imaging of single particles and biomolecules. J. Struct. Biol. 144, 219–227 (2003).
Loh, D. & Elser, V. Reconstruction algorithm for single-particle diffraction imaging experiments. Phys. Rev. E 80, 1–20 (2009).
Maia, F. R. N. C., Ekeberg, T., Tımneanu, N., van der Spoel, D. & Hajdu, J. Structural variability and the incoherent addition of scattered intensities in single-particle diffraction. Phys. Rev. E 80, 031905 (2009).
Fischer, N., Konevega, A. L., Wintermeyer, W., Rodnina, M. V. & Stark, H. Ribosome dynamics and tRNA movement by time-resolved electron cryomicroscopy. Nature 466, 329–333 (2010).
Sander, B., Golas, M. M., Luhrmann, R. & Stark, H. An approach for de novo structure determination of dynamic molecular assemblies by electron cryomicroscopy. Structure 18, 667–676 (2010).
Elmlund, D. & Elmlund, H. SIMPLE: Software for ab initio reconstruction of heterogeneous single-particles. J. Struct. Biol. 180, 420–427 (2012).
Emma, P. et al. First lasing and operation of an ångstrom-wavelength free-electron laser. Nature Photon. 4, 641–647 (2010).
Shively, J. M., Ball, F., Brown, D. H. & Saunders, R. E. Functional organelles in prokaryotes: polyhedral inclusions (carboxysomes) of Thiobacillus neapolitanus. Science 182, 584–586 (1973).
Rae, B. D., Long, B. M., Badger M. R. & Price, G. D. Functions, compositions, and evolution of the two types of carboxysomes: polyhedral microcompartments that facilitate CO2 fixation in cyanobacteria and some proteobacteria. Microbiol. Mol. Biol. Rev. 77, 357–379 (2013).
Iancu, C. V. et al. The structure of isolated Synechococcus strain WH8102 carboxysomes as revealed by electron cryotomography. J. Mol. Biol. 372, 764–773 (2007).
Espie, G. S. & Kimber, M. S. Carboxysomes: cyanobacterial Rubisco comes in small packages. Photosynth. Res. 109, 7–20 (2011).
Bozek, J. D. AMO instrumentation for the LCLS X-ray FEL. Eur. Phys. J. Spec. Top. 169, 129–132 (2009).
Bostedt, C. et al. Ultra-fast and ultra-intense X-ray sciences: first results from the Linac Coherent Light Source free-electron laser. J. Phys. B 46, 164003 (2013).
DePonte, D. P. Gas dynamic virtual nozzle for generation of microscopic droplet streams. J. Phys. D 41, 195505 (2008).
Murphy, W. K. & Sears, G. W. Production of particulate beams. J. Appl. Phys. 35,1986–1987 (1964).
Bogan, M. J. et al. Single particle X-ray diffractive imaging. Nano Lett. 8, 310–316 (2008).
Strüder, L. et al. Large-format, high-speed, X-ray pnCCDs combined with electron and ion imaging spectrometers in a multipurpose chamber for experiments at 4th generation light sources. Nucl. Instrum. Meth. Phys. Res. A 614, 483–496 (2010).
Miao, J., Sayre, D. & Chapman, H. N. Phase retrieval from the magnitude of the Fourier transforms of nonperiodic objects. J. Opt. Soc. Am. A 15, 1662–1669 (1998).
Miao, J. et al. Quantitative image reconstruction of GaN quantum dots from oversampled diffraction intensities alone. Phys. Rev. Lett. 95, 085503 (2005).
Thibault, P., Elser, V., Jacobsen, C., Shapiro, D. & Sayre, D. Reconstruction of a yeast cell from X-ray diffraction data. Acta Crystallogr. A 62, 248–261 (2006).
Kassemeyer, S. et al. Femtosecond free-electron laser x-ray diffraction data sets for algorithm development. Opt. Express 20, 4149–4158 (2012).
Van der Spoel, D., Marklund, E. G., Larsson, D. S. D. & Caleman, C. Proteins, lipids, and water in the gas phase. Macromol. Biosci. 11, 50–59 (2011).
Rouse, S. L., Marcoux, J., Robinson, C. V. & Sansom, M. S. P. Dodecyl maltoside protects membrane proteins in vacuo. Biophys. J. 105, 648–656 (2013).
Tito, M. A., Tars, K., Valegård, K., Hajdu, J. & Robinson, C. V. Electrospray time-of-flight mass spectrometry of the intact MS2 virus capsid. J. Am. Chem. Soc. 122, 3550–3551 (2000).
Maia, F. R. N. C., Ekeberg, T., van der Spoel, D. & Hajdu, J. Hawk: the image reconstruction package for coherent X-ray diffractive imaging. J. Appl. Crystallogr. 43, 1535–1539 (2010).
Chapman, H. N. et al. High-resolution ab initio three-dimensional X-ray diffraction microscopy. J. Opt. Soc. Am. A 23, 1179–1200 (2006).
Saxton, W. O. & Baumeister, W. The correlation averaging of a regularly arranged bacterial cell envelope protein. J. Microsc. 127, 127–138 (1982).
Van Heel, M., Keegstra, W., Schutter, W. & van Brüggen E. F. J. in Structure and Function of Invertebrate Respiratory Proteins, EMBO Workshop 1982, Life Chemistry Reports (ed. Wood, E. J.) Suppl. 1, 69–73 (1982).
Van Heel, M. & Schatz, M. Fourier shell correlation threshold criteria. J. Struct. Biol. 151, 250–262 (2005).
Barty, A. et al. A new resource for processing serial X-ray diffraction data. J. Appl. Cryst. 47, 1118–1131 (2014).
Thibault, P. Algorithmic Methods in Diffraction Microscopy PhD thesis, Cornell Univ. (2007).
Maia, F. R. N. C. The coherent X-ray imaging data bank. Nature Methods 9, 854–855 (2012).
Hamzeh, F. M. & Bragg, R. H. Small angle scattering of X-rays from groups of nonrandomly oriented ellipsoids of revolution of low concentration. J. Appl. Phys. 45, 3189–3195 (1974).
Acknowledgements
This work was supported by the Swedish Research Council, the Knut and Alice Wallenberg Foundation, the European Research Council, the Röntgen-Ångström Cluster and Stiftelsen Olle Engkvist Byggmästare. Portions of this research were carried out at the Linac Coherent Light Source, a national user facility operated by Stanford University on behalf of the US Department of Energy, Office of Basic Energy Sciences. The authors thank the scientific and technical staff of the LCLS for support. The authors thank the CAMP collaboration for giving access to their experimental set-up and for supporting the experiment at the LCLS. The authors also acknowledge the Max Planck Society for funding the development and operation of the CAMP instrument.
Author information
Authors and Affiliations
Contributions
J.H., I.A., M.F.H., F.R.N.C.M. and T.E. developed the imaging concept and conceived the experiment. M.F.H., F.R.N.C.M., T.E., A.B., N.D.L., A.M., G.V.D.S. and D.L. developed ideas and software to process the diffraction data. D.H., K.J., G.H.C., M.S., M.I. and I.A. prepared and characterized carboxysomes for the study. J.H., B.I., D.P.D., R.A.K., M.S., J.A., M.M.S. and D.W. developed and operated the sample injector. J.D.B., C.B., S.C., N.T., M.S. and M.M.S. operated the beamline at the LCLS. R.H. and N.K. operated the pnCCD detectors. M.F.H., F.R.N.C.M., T.E., N.D.L., G.V.D.S., A.B., J.A., M.M.S., M.S., M.L., F.S., D.R., A.R., S.E., H.N.C. and J.H. characterized the imaging apparatus and carried out the experiment. M.F.H., F.R.N.C.M. and T.E. processed the data. M.F.H., F.R.N.C.M., I.A. and J.H. analysed the results and wrote the manuscript with input from the others.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 618 kb)
Rights and permissions
About this article
Cite this article
Hantke, M., Hasse, D., Maia, F. et al. High-throughput imaging of heterogeneous cell organelles with an X-ray laser. Nature Photon 8, 943–949 (2014). https://doi.org/10.1038/nphoton.2014.270
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphoton.2014.270
This article is cited by
-
First commissioning results of the coherent scattering and imaging endstation at the Shanghai soft X-ray free-electron laser facility
Nuclear Science and Techniques (2022)
-
An encryption–decryption framework to validating single-particle imaging
Scientific Reports (2021)
-
Methods and application of coherent X-ray diffraction imaging of noncrystalline particles
Biophysical Reviews (2020)
-
The role of transient resonances for ultra-fast imaging of single sucrose nanoclusters
Nature Communications (2020)
-
Diffraction data from aerosolized Coliphage PR772 virus particles imaged with the Linac Coherent Light Source
Scientific Data (2020)