Abstract
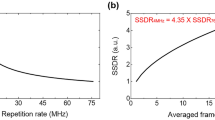
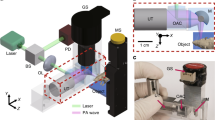
Multiphoton microscopy (MPM) is widely used in vivo for optical sectioning deep inside scattering tissue1,2. Phosphorescence lifetime imaging microscopy (PLIM)3 is a powerful technique for obtaining biologically relevant chemical information through Förster resonance energy transfer and phosphorescence quenching4,5. Point-measurement PLIM6 of phosphorescence quenching probes has recently provided oxygen partial pressure measurements in small rodent brain vasculature identified by high-resolution MPM7,8. However, the maximum fluorescence generation rate, which is inversely proportional to the phosphorescence lifetime, fundamentally limits PLIM pixel rates. Here, we demonstrate experimentally a parallel-excitation/parallel-collection MPM–PLIM system that increases the pixel rate by a factor of 100 compared with conventional configurations, while simultaneously acquiring lifetime and intensity images at depth in vivo. Full-frame, three-dimensional, in vivo PLIM imaging of phosphorescent quenching dye is presented for the first time and defines a new platform for biological and medical imaging.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Denk, W., Strickler, J. H. & Webb, W. W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990).
Kobat, D., Horton, N. G. & Xu, C. In vivo two-photon microscopy to 1.6-mm depth in mouse cortex. J. Biomed. Opt. 16, 106014 (2011).
Draaijer, A., Sanders, R. & Gerristen, H. C. in Handbook of Biological Confocal Microscopy (ed. Pawley, J. B.) 491–505 (Plenum Press, 1995).
Wallrabe, H. & Periasamy, A. Imaging protein molecules using FRET and FLIM microscopy. Curr. Opin. Biotechnol. 16, 19–27 (2004).
Zhong, W., Urayama, P. & Mycek, M. A. Imaging fluorescence lifetime modulation of a ruthenium-based dye in living cells: the potential for oxygen sensing. J. Phys. D 36, 1689–1695 (2003).
Gratton, E., Breusegem, S., Sutin, J., Ruan, Q. & Barry, N. Fluorescence lifetime imaging for the two-photon microscope: time-domain and frequency-domain methods. J. Biomed. Opt. 8, 381–390 (2003).
Sakadžić, S. et al. Two-photon high-resolution measurement of partial pressure of oxygen in cerebral vasculature and tissue. Nature Methods 7, 755–759 (2010).
Lecoq, J. et al. Simultaneous two-photon imaging of oxygen and blood flow in deep cerebral vessels. Nature Med. 17, 893–898 (2011).
Brakenhoff, G. J. et al. Real-time two-photon confocal microscopy using a femtosecond, amplified Ti:sapphire system. J. Microsc. 181, 253–259 (1996).
Buist, A. H., Muller, M., Squier, J. & Brakenhoff, G. J. Real-time two-photon absorption microscopy using multipoint excitation. J. Microsc. 192, 217–226 (1998).
Fricke, M. & Nielsen, T. Two-dimensional imaging without scanning by multifocal multiphoton microscopy. Appl. Opt. 44, 2984–2988 (2005).
Li, D. D-U. et al. Time-domain fluorescence lifetime imaging techniques suitable for solid-state imaging sensor arrays. Sensors 12, 5650–5669 (2012).
Li, D. U. et al. Real-time fluorescence lifetime imaging system with a 32 × 32 0.13 µm CMOS low dark-count single-photon avalanche diode array. Opt. Express 18, 10257–10269 (2010).
Li, D. U. et al. Video-rate fluorescence lifetime imaging camera with CMOS single-photon avalanche diode arrays and high-speed imaging algorithm. J. Biomed. Opt. 16, 096012 (2011).
Hartmann, P. & Ziegler, W. Lifetime imaging of luminescent oxygen sensors based on all-solid-state technology. Anal. Chem. 68, 4512–4514 (1996).
Sakadžić, S. et al. Simultaneous imaging of cerebral partial pressure of oxygen and blood flow during functional activation and cortical spreading depression. Appl. Opt. 48, D169–D177 (2009).
Dunphy, I., Vinogradov, S. A. & Wilson, D. F. Oxyphor R2 and G2: phosphors for measuring oxygen by oxygen-dependent quenching of phosphorescence. Anal. Biochem. 310, 191–198 (2002).
Morris, K. J., Roach, M. S., Xu, W., Demas, J. N. & DeGraff, B. A. Luminescence lifetime standards for the nanosecond to microsecond range and oxygen quenching of ruthenium (II) complexes. Anal. Chem. 89, 9310–9314 (2007).
Finikova, O. S. et al. Oxygen microscopy by two-photon-excited phosphorescence. ChemPhysChem 9, 1673–1679 (2008).
Szmacinski, H., Gryczynski, I. & Lakowicz, J. R. Spatially localized ballistic two-photon excitation in scattering media. Biospectroscopy 4, 303–310 (1998).
Theer, P. & Denk, W. On the fundamental imaging-depth limit in two-photon microscopy. J. Opt. Soc. Am. A 23, 3139–3149 (2006).
Sanders, J. S., Driggers, R. G., Halford, C. E. & Griffin, S. T. Imaging with frequency-modulated reticles. Opt. Eng. 30, 1720–1724 (1991).
Howard, S. S., Straub, A. A. & Xu, C. Multiphoton modulation microscopy for high-speed deep biological imaging. IEEE/OSA Conf. Lasers Electro-Optics (CLEO), CWD6 (2010).
Futia, G., Schlup, P., Winters, D. G. & Bartels, R. A. Spatially-chirped modulation imaging of absorption and fluorescent objects on single-element optical detector. Opt. Express 19, 1626–1640 (2011).
Philip, J. & Carlsson, K. Theoretical investigation of the signal-to-noise ratio in fluorescence lifetime imaging. J. Opt. Soc. Am. A 20, 368–379 (2003).
Redford, G. I. & Clegg, R. M. Polar plot representation for frequency-domain analysis of fluorescence lifetimes. J. Fluoresc. 15, 805–815 (2005).
Nezam, S. M. R. M. High-speed polygon-scanner-based wavelength-swept laser source in the telescope-less configurations with application in optical coherence tomography. Opt. Lett. 33, 1741–1743 (2008).
Chen, M., Ding, Z., Wang, L., Wu, T. & Wang, B. Wavelength-swept laser around 1300 nm based on polygon filter in Littrow telescope-less configuration. Proc. SPIE 7845, 78452K (2010).
Intaglietta, M., Johnson, P. C. & Winslow, R. M. Microvascular and tissue oxygen distribution. Cardiovasc. Res. 32, 632–643 (1996).
Maurin, M., Stephan, O., Vial, J. C., Marder, S. R. & van der Sanden, B. Deep in vivo two-photon imaging of blood vessels with a new dye encapsulated in pluronic nanomicelles. J. Biomed. Opt. 16, 036001 (2011).
Acknowledgements
This research project was supported in part by the National Science Foundation (NSF)/Division of Biological Infrastructure (DBI) (grant no. DBI-0546227) and the National Institutes of Health (NIH)/National Cancer Institute (NCI) (grant no. R01-CA133148). Nicholas G. Horton was supported by an NSF Graduate Research Fellowship (grant no. DGE-0707428). The authors would like to thank C. Schafer (Cornell University) and S. Vinogradov (Department of Biochemistry and Biophysics, University of Pennsylvania) for their helpful discussions on brain imaging and phosphorescence quenching dyes for in vivo oxygen sensing, respectively.
Author information
Authors and Affiliations
Contributions
S.H. coordinated the project, designed and built the microscope, designed and fabricated the linear SLM, wrote control and image-processing software, performed simulations, wrote analysis algorithms, analysed data, carried out animal preparation and surgery, prepared dye and calibrations, and wrote the manuscript. A.S. greatly assisted with microscope design and assembly, performed simulations and analysis, wrote analysis algorithms, significantly contributed to the content of the manuscript, and performed experiments verifying pixel rate increase. N.H. and D.K. assisted in animal preparation and surgery for imaging. C.X. initiated the project, significantly contributed to the design of M4 and experimental design, and greatly contributed to the theoretical and experimental discussions. All authors contributed to editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary video
Supplementary video (MOV 165 kb)
Supplementary video
Supplementary video (MOV 98 kb)
Supplementary video
Supplementary video (MOV 109 kb)
Rights and permissions
About this article
Cite this article
Howard, S., Straub, A., Horton, N. et al. Frequency-multiplexed in vivo multiphoton phosphorescence lifetime microscopy. Nature Photon 7, 33–37 (2013). https://doi.org/10.1038/nphoton.2012.307
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphoton.2012.307
This article is cited by
-
Fast wide-field upconversion luminescence lifetime thermometry enabled by single-shot compressed ultrahigh-speed imaging
Nature Communications (2021)
-
Parallelized volumetric fluorescence microscopy with a reconfigurable coded incoherent light-sheet array
Light: Science & Applications (2020)
-
Mapping O2 concentration in ex-vivo tissue samples on a fast PLIM macro-imager
Scientific Reports (2020)
-
Merger of dynamic two-photon and phosphorescence lifetime microscopy reveals dependence of lymphocyte motility on oxygen in solid and hematological tumors
Journal for ImmunoTherapy of Cancer (2019)
-
Imaging intratumoral metabolic heterogeneity
Nature Biomedical Engineering (2019)