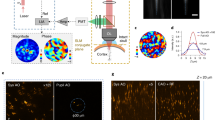
Abstract
A long-standing goal in biomedical imaging, the control of light inside turbid media, requires knowledge of how the phase and amplitude of an illuminating wavefront are transformed as the electric field propagates inside a scattering sample onto a target plane. So far, it has proved challenging to non-invasively characterize the scattered optical wavefront inside a disordered medium. Here, we present a non-invasive scattering compensation method, termed F-SHARP, which allows us to measure the scattered electric-field point spread function (E-field PSF) in three dimensions. Knowledge of the phase and amplitude of the E-field PSF makes it possible to optically cancel sample turbulence. We demonstrate the imaging capabilities of this technique on a variety of samples and notably through vertebrate brains and across thinned skull in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Denk, W., Strickler, J. & Webb, W. Two-photon laser scanning fluorescence microscopy. Science 248, 73–76 (1990).
Helmchen, F. & Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2, 932–940 (2005).
Ntziachristos, V. Going deeper than microscopy: the optical imaging frontier in biology. Nat. Methods 7, 603–614 (2010).
Booth, M. J. Adaptive optical microscopy: the ongoing quest for a perfect image. Light: Sci. Appl. 3, e165 (2014).
Tao, X. et al. Adaptive optics confocal microscopy using direct wavefront sensing. Opt. Lett. 36, 1062–1064 (2011).
Ji, N., Milkie, D. E. & Betzig, E. Adaptive optics via pupil segmentation for high-resolution imaging in biological tissues. Nat. Methods 7, 141–147 (2009).
Wang, C. et al. Multiplexed aberration measurement for deep tissue imaging in vivo. Nat. Methods 11, 1037–1040 (2014).
Olivier, N., Débarre, D. & Beaurepaire, E. Dynamic aberration correction for multiharmonic microscopy. Opt. Lett. 34, 3145–3147 (2009).
Wang, K. et al. Rapid adaptive optical recovery of optimal resolution over large volumes. Nat. Methods 11, 625–628 (2014).
Booth, M. J., Neil, M. A. A., Juškaitis, R. & Wilson, T. Adaptive aberration correction in a confocal microscope. Proc. Natl Acad. Sci. USA 99, 5788–5792 (2002).
Débarre, D. et al. Image-based adaptive optics for two-photon microscopy. Opt. Lett. 34, 2495–2497 (2009).
Yaqoob, Z., Psaltis, D., Feld, M. S. & Yang, C. Optical phase conjugation for turbidity suppression in biological samples. Nat. Photon. 2, 110–115 (2008).
Hsieh, C. L., Pu, Y., Grange, R. & Psaltis, D. Digital phase conjugation of second harmonic radiation emitted by nanoparticles in turbid media. Opt. Express 18, 12283–12290 (2010).
Cui, M. & Yang, C. Implementation of a digital optical phase conjugation system and its application to study the robustness of turbidity suppression by phase conjugation. Opt. Express 18, 3444–3455 (2010).
Vellekoop, I. M. & Mosk, A. P. Focusing coherent light through opaque strongly scattering media. Opt. Lett. 32, 2309–2311 (2007).
Popoff, S. M. et al. Measuring the transmission matrix in optics: an approach to the study and control of light propagation in disordered media. Phys. Rev. Lett. 104, 100601 (2010).
Choi, W., Mosk, A. P., Park, Q. H., Choi, W. & Choi, W. Transmission eigenchannels in a disordered medium. Phys. Rev. B 83, 134207 (2011).
Hsieh, C. L., Pu, Y., Grange, R., Laporte, G. & Psaltis, D. Imaging through turbid layers by scanning the phase conjugated second harmonic radiation from a nanoparticle. Opt. Express 18, 20723–20731 (2010).
Vellekoop, I. M. & Aegerter, C. M. Scattered light fluorescence microscopy: imaging through turbid layers. Opt. Lett. 35, 1245–1247 (2010).
Vellekoop, I. M., Lagendijk, A. & Mosk, A. P. Exploiting disorder for perfect focusing. Nat. Photon. 4, 320–322 (2010).
Katz, O., Small, E. & Silberberg, Y. Looking around corners and through thin turbid layers in real time with scattered incoherent light. Nat. Photon. 6, 549–553 (2012).
Guan, Y., Katz, O., Small, E., Zhou, J. & Silberberg, Y. Polarization control of multiply scattered light through random media by wavefront shaping. Opt. Lett. 37, 4663–4665 (2012).
Katz, O., Small, E., Bromberg, Y. & Silberberg, Y. Focusing and compression of ultrashort pulses through scattering media. Nat. Photon. 5, 372–377 (2011).
Aulbach, J., Gjonaj, B., Johnson, P. M., Mosk, A. P. & Lagendijk, A. Control of light transmission through opaque scattering media in space and time. Phys. Rev. Lett. 106, 103901 (2011).
Horstmeyer, R., Ruan, H. & Yang, C. Guidestar-assisted wavefront-shaping methods for focusing light into biological tissue. Nat. Photon. 9, 563–571 (2015).
Xu, X., Liu, H. & Wang, L. V. Time-reversed ultrasonically encoded optical focusing into scattering media. Nat. Photon. 5, 154–157 (2011).
Si, K., Fiolka, R. & Cui, M. Fluorescence imaging beyond the ballistic regime by ultrasound-pulse-guided digital phase conjugation. Nat. Photon. 6, 657–661 (2012).
Wang, Y. M., Judkewitz, B., Dimarzio, C. A. & Yang, C. Deep-tissue focal fluorescence imaging with digitally time-reversed ultrasound-encoded light. Nat. Commun. 3, 928 (2012).
Judkewitz, B., Wang, Y. M., Horstmeyer, R., Mathy, A. & Yang, C. Speckle-scale focusing in the diffusive regime with time reversal of variance-encoded light (TROVE). Nat. Photon. 7, 300–305 (2013).
Chaigne, T. et al. Controlling light in scattering media non-invasively using the photoacoustic transmission matrix. Nat. Photon. 8, 58–64 (2013).
Lai, P., Wang, L., Tay, J. W. & Wang, L. V. Photoacoustically guided wavefront shaping for enhanced optical focusing in scattering media. Nat. Photon. 9, 126–132 (2015).
Aviles-Espinosa, R. et al. Measurement and correction of in vivo sample aberrations employing a nonlinear guide-star in two-photon excited fluorescence microscopy. Biomed. Opt. Express 2, 3135–3149 (2011).
Tang, J., Germain, R. N. & Cui, M. Superpenetration optical microscopy by iterative multiphoton adaptive compensation technique. Proc. Natl Acad. Sci. USA 109, 8434–8439 (2012).
Katz, O., Small, E., Guan, Y. & Silberberg, Y. Noninvasive nonlinear focusing and imaging through strongly scattering turbid layers. Optica 1, 170–174 (2014).
Kong, L. & Cui, M. In vivo fluorescence microscopy via iterative multi-photon adaptive compensation technique. Opt. Express 22, 23786–23794 (2014).
Park, J. H., Sun, W. & Cui, M. High-resolution in vivo imaging of mouse brain through the intact skull. Proc. Natl Acad. Sci. USA 112, 9236–9241 (2015).
Göppert-Mayer, M. Über Elementarakte mit zwei Quantensprüngen. Ann. Phys. 401, 273–294 (1931).
Gabor, D. A new microscopic principle. Nature 161, 777–778 (1948).
Yamaguchi, I. & Zhang, T. Phase-shifting digital holography. Opt. Lett. 22, 1268–1270 (1997).
Freund, I., Rosenbluh, M. & Feng, S. Memory effects in propagation of optical waves through disordered media. Phys. Rev. Lett. 61, 2328–2331 (1988).
Feng, S., Kane, C., Lee, P. & Stone, A. Correlations and fluctuations of coherent wave transmission through disordered media. Phys. Rev. Lett. 61, 834–837 (1988).
Judkewitz, B., Horstmeyer, R., Vellekoop, I. M., Papadopoulos, I. N. & Yang, C. Translation correlations in anisotropically scattering media. Nat. Phys. 11, 684–689 (2015).
Schott, S., Bertolotti, J., Léger, J. F., Bourdieu, L. & Gigan, S. Characterization of the angular memory effect of scattered light in biological tissues. Opt. Express 23, 13505–13516 (2015).
Rupprecht, P., Prendergast, A., Wyart, C. & Friedrich, R. W. Remote z-scanning with a macroscopic voice coil motor for fast 3D multiphoton laser scanning microscopy. Biomed. Opt. Express 7, 1656–1616 (2016).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nat. Photon. 499, 295–300 (2013).
Tamamaki, N. et al. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J. Comp. Neurol. 467, 60–79 (2003).
Feng, G. et al. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28, 41–51 (2000).
Campagnola, P. J. & Loew, L. M. Second-harmonic imaging microscopy for visualizing biomolecular arrays in cells, tissues and organisms. Nat. Biotechnol. 21, 1356–1360 (2003).
Débarre, D. et al. Imaging lipid bodies in cells and tissues using third-harmonic generation microscopy. Nat. Methods 3, 47–53 (2006).
Freudiger, C. W. et al. Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science 322, 1857–1861 (2008).
Zumbusch, A., Holtom, G. R. & Xie, X. S. Three-dimensional vibrational imaging by coherent anti-Stokes Raman scattering. Phys. Rev. Lett. 82, 4142 (1999).
Horton, N. G. et al. In vivo three-photon microscopy of subcortical structures within an intact mouse brain. Nat. Photon. 7, 205–209 (2013).
Acknowledgements
The authors thank R. Horstmeyer for discussions and help with the manuscript and S. Smith, A. Packer, C. Rowlands, E. Bobrov and M. Hoffmann for critically reviewing the manuscript. The authors thank L. Schulze for help with the zebrafish larvae mounting protocol and N. Hakiy for zebrafish husbandry. The authors also thank C. Wyart and A. Prendergast for providing the NeuroD:GCaMP6f zebrafish line. This work was supported by the DFG (EXC 257 NeuroCure), the European Research Council (ERC-2015-CoG-682422, to J.F.A.P.) and the European Union (3x3Dimaging 323945, to J.F.A.P.). I.N.P. is a recipient of the Early-PostDoc mobility fellowship of the Swiss National Science Foundation.
Author information
Authors and Affiliations
Contributions
B.J. and I.N.P. conceived and developed the idea. I.N.P. designed experiments with help from B.J. I.N.P. built the set-up, collected and analysed data. J.-S.J. performed mouse procedures under supervision from J.F.A.P. I.N.P. and B.J. wrote the manuscript. B.J. supervised the project.
Corresponding author
Ethics declarations
Competing interests
I.N.P. and B.J. have submitted a patent application on part of the described work.
Supplementary information
Supplementary information
Supplementary information (PDF 3409 kb)
Supplementary information
Supplementary Movie 1 (AVI 2443 kb)
Supplementary information
Supplementary Movie 2 (AVI 1455 kb)
Supplementary information
Supplementary Movie 3 (AVI 380 kb)
Rights and permissions
About this article
Cite this article
Papadopoulos, I., Jouhanneau, JS., Poulet, J. et al. Scattering compensation by focus scanning holographic aberration probing (F-SHARP). Nature Photon 11, 116–123 (2017). https://doi.org/10.1038/nphoton.2016.252
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nphoton.2016.252
This article is cited by
-
Construction and use of an adaptive optics two-photon microscope with direct wavefront sensing
Nature Protocols (2023)
-
Robust and adjustable dynamic scattering compensation for high-precision deep tissue optogenetics
Communications Biology (2023)
-
Recent advances in optical imaging through deep tissue: imaging probes and techniques
Biomaterials Research (2022)
-
Deep tissue multi-photon imaging using adaptive optics with direct focus sensing and shaping
Nature Biotechnology (2022)
-
Bacteria eat nanoprobes for aggregation-enhanced imaging and killing diverse microorganisms
Nature Communications (2022)