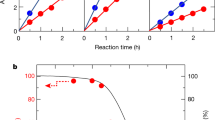
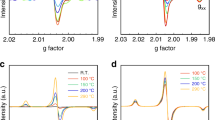
Abstract
The generation of hydrogen from water using sunlight could potentially form the basis of a clean and renewable source of energy. Various water-splitting methods have been investigated previously1,2,3,4,5,6,7,8, but the use of photocatalysts to split water into stoichiometric amounts of H2 and O2 (overall water splitting) without the use of external bias or sacrificial reagents is of particular interest because of its simplicity and potential low cost of operation1,2,3,4. However, despite progress in the past decade, semiconductor water-splitting photocatalysts (such as (Ga1−xZnx)(N1−xOx)) do not exhibit good activity beyond 440 nm (refs 1,2,9) and water-splitting devices that can harvest visible light typically have a low solar-to-hydrogen efficiency of around 0.1%6,7. Here we show that cobalt(II) oxide (CoO) nanoparticles can carry out overall water splitting with a solar-to-hydrogen efficiency of around 5%. The photocatalysts were synthesized from non-active CoO micropowders using two distinct methods (femtosecond laser ablation and mechanical ball milling), and the CoO nanoparticles that result can decompose pure water under visible-light irradiation without any co-catalysts or sacrificial reagents. Using electrochemical impedance spectroscopy, we show that the high photocatalytic activity of the nanoparticles arises from a significant shift in the position of the band edge of the material.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Chen, X. B., Shen, S. H., Guo, L. J. & Mao, S. S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 110, 6503–6570 (2010).
Kudo, A. & Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 38, 253–278 (2009).
Osterloh, F. E. & Parkinson, B. A. Recent developments in solar water-splitting photocatalysis. Mater. Res. Soc. Bull. 36, 17–22 (2011).
Mallouk, T. E. The emerging technology of solar fuels. J. Phys. Chem. Lett. 1, 2738–2739 (2010).
Walter, M. G. et al. Solar water splitting cells. Chem. Rev. 110, 6446–6473 (2010).
Mubeen, S. et al. An autonomous photosynthetic device in which all charge carriers derive from surface plasmons. Nature Nanotech. 8, 247–251 (2013).
Liu, C., Tang, J., Chen, H. M., Liu, B. & Yang, P. A fully integrated nanosystem of semiconductor nanowires for direct solar water splitting. Nano Lett. 13, 2989–2992 (2013).
Nocera, D. G. The artificial leaf. Acc. Chem. Res. 45, 767–776 (2012).
Maeda, K., Teramura, K. & Domen, K. Effect of post-calcination on photocatalytic activity of (Ga1–xZnx)(N1–xOx) solid solution for overall water splitting under visible light. J. Catal. 254, 198–204 (2008).
Shi, H. G. & He, X. M. Large-scale synthesis and magnetic properties of cubic CoO nanoparticles. J. Phys. Chem. Solids 73, 646–650 (2012).
Yin, J. S. & Wang, Z. L. Ordered self-assembling of tetrahedral oxide nanocrystals. Phys. Rev. Lett. 79, 2570–2573 (1997).
Yang, H. M., Ouyang, J. & Tang, A. D. Single step synthesis of high-purity CoO nanocrystals. J. Phys. Chem. B 111, 8006–8013 (2007).
Barreca, D. et al. Controlled vapor-phase synthesis of cobalt oxide nanomaterials with tuned composition and spatial organization. CrystEngComm 12, 2185–2197 (2010).
Gallant, D., Pezolet, M. & Simard, S. Optical and physical properties of cobalt oxide films electrogenerated in bicarbonate aqueous media. J. Phys. Chem. B 110, 6871–6880 (2006).
Grimblot, J., Bonnelle, J. P. & Beaufils, J. P. ESCA study on cobalt and molybdenum deposited on alumina – quantitative-analysis of recovery and states of specific cation adsorption. J. Electron Spectrosc. Relat. Phenom. 8, 437–447 (1976).
Amirav, L. & Alivisatos, A. P. Photocatalytic hydrogen production with tunable nanorod heterostructures. J. Phys. Chem. Lett. 1, 1051–1054 (2010).
Xu, Y. & Schoonen, M. A. A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Miner. 85, 543–556 (2000).
Vayssieres, L. et al. One-dimensional quantum-confinement effect in α-Fe2O3 ultrafine nanorod arrays. Adv. Mater. 17, 2320–2323 (2005).
Wang, H. L. & Turner, J. A. Characterization of hematite thin films for photoelectrochemical water splitting in a dual photoelectrode device. J. Electrochem. Soc. 157, F173–F178 (2010).
Markus, T. Z. et al. Electronic structure of CdSe nanoparticles adsorbed on Au electrodes by an organic linker: Fermi level pinning of the HOMO. J. Phys. Chem. C 113, 14200–14206 (2009).
Frame, F. A. et al. First demonstration of CdSe as a photocatalyst for hydrogen evolution from water under UV and visible light. Chem. Commun. 2206–2208 (2008).
McCarthy, T. J., Tanzer, T. A. & Kanatzidis, M. G. A new metastable 3-dimensional bismuth sulfide with large tunnels – synthesis, structural characterization, ion-exchange properties, and reactivity of KBi3S5 . J. Am. Chem. Soc. 117, 1294–1301 (1995).
Ishikawa, A. et al. Oxysulfide Sm2Ti2S2O5 as a stable photocatalyst for water oxidation and reduction under visible light irradiation (λ ≤ 650 nm). J. Am. Chem. Soc. 124, 13547–13553 (2002).
Mane, A. U. & Shivashankar, S. A. MOCVD of cobalt oxide thin films: dependence of growth, microstructure, and optical properties on the source of oxidation. J. Cryst. Growth 254, 368–377 (2003).
Holmes, M. A., Townsend, T. K. & Osterloh, F. E. Quantum confinement controlled photocatalytic water splitting by suspended CdSe nanocrystals. Chem. Commun. 48, 371–373 (2012).
Johnson, C. A. et al. Visible-light photoconductivity of Zn1–xCoxO and its dependence on Co2+ concentration. Phys. Rev. B 84, 125203 (2011).
Jasieniak, J., Califano, M. & Watkins, S. E. Size-dependent valence and conduction band-edge energies of semiconductor nanocrystals. ACS Nano 5, 5888–5902 (2011).
Pinaud, B. A., Chen, Z. B., Abram, D. N. & Jaramillo, T. F. Thin films of sodium birnessite-type MnO2: optical properties, electronic band structure, and solar photoelectrochemistry. J. Phys. Chem. C 115, 11830–11838 (2011).
Matsumoto, Y. Energy positions of oxide semiconductors and photocatalysis with iron complex oxides. J. Solid State Chem. 126, 227–234 (1996).
Riha, S. C. et al. Atomic layer deposition of a submonolayer catalyst for the enhanced photoelectrochemical performance of water oxidation with hematite. ACS Nano 7, 2396–2405 (2013).
Marinescu, S. C., Winkler, J. R. & Gray, H. B. Molecular mechanisms of cobalt-catalyzed hydrogen evolution. Proc. Natl Acad. Sci. USA 109, 15127–15131 (2012).
Jiao, F. & Frei, H. Nanostructured cobalt oxide clusters in mesoporous silica as efficient oxygen-evolving catalysts. Angew. Chem. Int. Ed. 48, 1841–1844 (2009).
Acknowledgements
Financial support from the Robert A. Welch Foundation (E-1728), National Science Foundation (DMR 0907336) and Department of Energy (DE-FG02-13ER46917) is acknowledged. We thank J. Shan and P. Tian for assistance with the purchase of the femtosecond laser, M. Shen, J. Deng, R. Thummel, R. Zhong, H. Fang, Z. Tang, D. Achey, X. Ni, J. Sun, X. Ren, Y. Qi, S Pei, J. Zhao, P. Ruchhoeft, Q. Zhang and J. Allen for help and valuable discussions, and Z. Liu, X. Gong, P. Peng, P. Zhu and M. Fang for various contributions.
Author information
Authors and Affiliations
Contributions
L.L. and J.M.B. conceived, designed and performed the experiments, and analysed the data. D.W. and F.R-H. performed TEM studies, Q.Z. and Y.L. performed ultraviolet–visible measurements, S.B. and X.C. helped design and fabricate reaction chambers, and assisted in electrochemical studies, Q.Y, J.Z. and G.F. assisted in laser ablation, Z.R. and H.F. assisted in ball-milling synthesis, Z.S., Z.Z., Y.W. and X.L. assisted in mass spectrometry, gas chromatography, SEM studies and the solar-simulator set-up, J.M.B. wrote the manuscript and prepared the figures. All authors discussed the results and commented on the manuscript. J.M.B. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1230 kb)
Rights and permissions
About this article
Cite this article
Liao, L., Zhang, Q., Su, Z. et al. Efficient solar water-splitting using a nanocrystalline CoO photocatalyst. Nature Nanotech 9, 69–73 (2014). https://doi.org/10.1038/nnano.2013.272
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2013.272
This article is cited by
-
Photoelectrochemical Water Splitting by Using Nanomaterials: A Review
Journal of Electronic Materials (2024)
-
Epitaxially grown silicon-based single-atom catalyst for visible-light-driven syngas production
Nature Communications (2023)
-
Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting
Nature (2023)
-
Hydrovoltaic effect-enhanced photocatalysis by polyacrylic acid/cobaltous oxide–nitrogen doped carbon system for efficient photocatalytic water splitting
Nature Communications (2023)
-
2D indium-VA semiconductors: Promising photocatalysts with intrinsic electric fields for water splitting
Science China Materials (2023)