Abstract
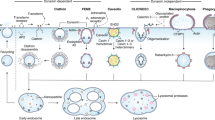
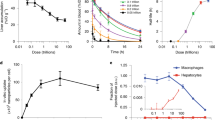
Nanoparticles are considered a primary vehicle for targeted therapies because they can pass biological barriers and enter and distribute within cells by energy-dependent pathways1,2,3. So far, most studies have shown that nanoparticle properties, such as size4,5,6 and surface7,8, can influence how cells internalize nanoparticles. Here, we show that uptake of nanoparticles by cells is also influenced by their cell cycle phase. Although cells in different phases of the cell cycle were found to internalize nanoparticles at similar rates, after 24 h the concentration of nanoparticles in the cells could be ranked according to the different phases: G2/M > S > G0/G1. Nanoparticles that are internalized by cells are not exported from cells but are split between daughter cells when the parent cell divides. Our results suggest that future studies on nanoparticle uptake should consider the cell cycle, because, in a cell population, the dose of internalized nanoparticles in each cell varies as the cell advances through the cell cycle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Kagan, V. E. et al. Fantastic voyage and opportunities of engineered nanomaterials: what are the potential risks of occupational exposures? J. Occup. Environ. Med. 52, 943–946 (2010).
Oberdörster, G. Safety assessment for nanotechnology and nanomedicine: concepts of nanotoxicology. J. Intern. Med. 267, 89–105 (2010).
Salvati, A. et al. Experimental and theoretical comparison of intracellular import of polymeric nanoparticles and small molecules: toward models of uptake kinetics. Nanomedicine http://dx.doi.org/10.1016/j.nano.2011.03.005 (2011).
Chithrani, B. D., Ghazani, A. A. & Chan, W. C. W. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 6, 662–668 (2006).
He, C. et al. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 31, 3657–3666 (2010).
Rejman, J., Oberle, V., Zuhorn, I. S. & Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 377, 159–169 (2004).
Lundqvist, M. et al. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl Acad. Sci. USA 105, 14265–14270 (2008).
Walczyk, D. et al. What the cell ‘sees’ in bionanoscience. J. Am. Chem. Soc. 132, 5761–5768 (2010).
Jiang, W., Kim, B. Y. S., Rutka, J. T. & Chan, W. C. W. Nanoparticle-mediated cellular response is size-dependent. Nature Nanotech. 3, 145–150 (2008).
Chithrani, B. D. & Chan, W. C. W. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 7, 1542–1550 (2007).
Gratton, S. E. A. et al. The effect of particle design on cellular internalization pathways. Proc. Natl Acad. Sci. USA 105, 11613–11618 (2008).
Cho, E. C., Au, L., Zhang, Q. & Xia, Y. The effects of size, shape, and surface functional group of gold nanostructures on their adsorption and internalization by cells. Small 6, 517–522 (2010).
Cho, E. C., Zhang, Q. & Xia, Y. The effect of sedimentation and diffusion on cellular uptake of gold nanoparticles. Nature Nanotech. 6, 385–391 (2011).
Lesniak, A. et al. Serum heat inactivation affects protein corona composition and nanoparticle uptake. Biomaterials 31, 9511–9518 (2010).
Xia, X.-R., Monteiro-Riviere, N. A. & Riviere, J. E. An index for characterization of nanomaterials in biological systems. Nature Nanotech. 5, 671–675 (2010).
Aggarwal, P. et al. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 61, 428–437 (2009).
Wilhelm, C. et al. Interaction of anionic superparamagnetic nanoparticles with cells: kinetic analyses of membrane adsorption and subsequent internalization. Langmuir 18, 8148–8155 (2002).
Cho, E. C., Xie, J., Wurm, P. A. & Xia, Y. Understanding the role of surface charges in cellular adsorption versus internalization by selectively removing gold nanoparticles on the cell surface with a I2/KI etchant. Nano Lett. 9, 1080–1084 (2009).
Lin, H.-C. et al. Quantitative measurement of nano-/microparticle endocytosis by cell mass spectrometry. Angew. Chem. Int. Ed. 49, 3460–3464 (2010).
Trono, J. D. et al. Size, concentration and incubation time dependence of gold nanoparticle uptake into pancreas cancer cells and its future application to X-ray drug delivery system. J. Radiat. Res. 52, 103–109 (2011).
Nel, A. E. et al. Understanding biophysicochemical interactions at the nano–bio interface. Nature Mater. 8, 543–557 (2009).
Colvin, V. L. The potential environmental impact of engineered nanomaterials. Nature Biotechnol. 21, 1166–1170 (2003).
Rivera Gil, P. et al. Correlating physico-chemical with toxicological properties of nanoparticles: the present and the future. ACS Nano 4, 5527–5531 (2010).
Errington, R. J. et al. Single cell nanoparticle tracking to model cell cycle dynamics and compartmental inheritance. Cell Cycle 9, 121–130 (2010).
Summers, H. D. et al. Statistical analysis of nanoparticle dosing in a dynamic cellular system. Nature Nanotech. 6, 170–174 (2011).
Hartwell, L. & Weinert, T. Checkpoints: controls that ensure the order of cell cycle events. Science 246, 629–634 (1989).
Hartwell, L. H., Culotti, J. & Reid, B. Genetic control of the cell-division cycle in yeast, I. Detection of mutants. Proc. Natl Acad. Sci. USA 66, 352–359 (1970).
Raucher, D. & Sheetz, M. P. Membrane expansion increases endocytosis rate during mitosis. J. Cell Biol. 144, 497–506 (1999).
Boucrot, E. & Kirchhausen, T. Endosomal recycling controls plasma membrane area during mitosis. Proc. Natl Acad. Sci. USA 104, 7939–7944 (2007).
Schweitzer, J. K., Burke, E. E., Goodson, H. V. & D'Souza-Schorey, C. Endocytosis resumes during late mitosis and is required for cytokinesis. J. Biol. Chem. 280, 41628–41635 (2005).
Snijder, B. et al. Population context determines cell-to-cell variability in endocytosis and virus infection. Nature 461, 520–523 (2009).
Bexiga, M. G. et al. Cationic nanoparticles induce caspase 3-, 7- and 9-mediated cytotoxicity in a human astrocytoma cell line. Nanotoxicology http://dx.doi.org/10.3109/17435390.2010.539713 (2011).
Shapero, K. et al. Time and space resolved uptake study of silica nanoparticles by human cells. Mol. BioSyst. 7, 371–378 (2011).
Steel, G. G. Growth Kinetics of Tumors: Cell Population Kinetics in Relation to the Growth and Treatment of Cancer (Oxford Univ. Press, 1977).
Fang, J., Nakamura, H. & Maeda, H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 63, 136–151 (2011).
Ruoslahti, E., Bhatia, S. N. & Sailor, M. J. Targeting of drugs and nanoparticles to tumors. J. Cell Biol. 188, 759–768 (2010).
Wang, M. & Thanou, M. Targeting nanoparticles to cancer. Pharmacol. Res. 62, 90–99 (2010).
Acknowledgements
Funding for the project was generously provided by the INSPIRE (Integrated NanoScience Platform for Ireland) programme, funded by the Irish Government's Programme for Research in Third Level Institutions, Cycle 4, National Development Plan 2007-2013 (J.A.K.), and the Irish Research Council for Science, Engineering and Technology (C.Å.), and is based on works supported by the Small Collaborative project NeuroNano funded by the European Commission 7th Framework Programme (NNP4-SL-2008-214547), Science Foundation Ireland (grant nos SFI/SRC/B1155 and 09/RFP/MTR2425) and the European Science Foundation Research Networking Programme EpitopeMap. A. Blanco (Conway Institute Flow Cytometry Facility, University College Dublin) is acknowledged for technical support with flow cytometry. Use of the Conway Institute Imaging Facility (University College Dublin) is also acknowledged. Endothelial hCMEC/D3 cells were provided by F. Miller and B.B. Wecksler (French National Institute of Health and Medical Research, Inserm, Paris, France).
Author information
Authors and Affiliations
Contributions
J.A.K. performed experiments, analysed and interpreted data, and wrote the paper. C.Å. developed the numerical simulations and analytical tools, analysed and interpreted data, and wrote the paper. A.S. supervised the experimental work, analysed and interpreted data, and wrote the paper. K.A.D. interpreted data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 3851 kb)
Supplementary information
Supplementary information (PPTX 367 kb)
Rights and permissions
About this article
Cite this article
Kim, J., Åberg, C., Salvati, A. et al. Role of cell cycle on the cellular uptake and dilution of nanoparticles in a cell population. Nature Nanotech 7, 62–68 (2012). https://doi.org/10.1038/nnano.2011.191
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2011.191
This article is cited by
-
Cu-doped TiO2 nanoparticles improve local antitumor immune activation and optimize dendritic cell vaccine strategies
Journal of Nanobiotechnology (2023)
-
Hepatocellular Carcinoma cells: activity of Amygdalin and Sorafenib in Targeting AMPK /mTOR and BCL-2 for anti-angiogenesis and apoptosis cell death
BMC Complementary Medicine and Therapies (2023)
-
Dual enhancement in the radiosensitivity of prostate cancer through nanoparticles and chemotherapeutics
Cancer Nanotechnology (2023)
-
Protein nanoparticle cellular fate and responses in murine macrophages
NPG Asia Materials (2023)
-
Endosomal sorting results in a selective separation of the protein corona from nanoparticles
Nature Communications (2023)