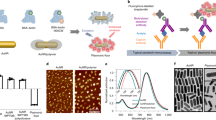
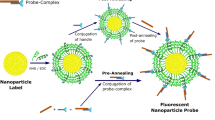
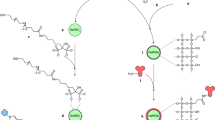
Abstract
Nanoparticles have emerged as key materials for biomedical applications because of their unique and tunable physical properties, multivalent targeting capability, and high cargo capacity1,2. Motivated by these properties and by current clinical needs, numerous diagnostic3,4,5,6,7,8,9,10 and therapeutic11,12,13 nanomaterials have recently emerged. Here we describe a novel nanoparticle targeting platform that uses a rapid, catalyst-free cycloaddition as the coupling mechanism. Antibodies against biomarkers of interest were modified with trans-cyclooctene and used as scaffolds to couple tetrazine-modified nanoparticles onto live cells. We show that the technique is fast, chemoselective, adaptable to metal nanomaterials, and scalable for biomedical use. This method also supports amplification of biomarker signals, making it superior to alternative targeting techniques including avidin/biotin.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Davis, M. E., Chen, Z. G. & Shin, D. M. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nature Rev. Drug Discov. 7, 771–782 (2008).
Weissleder, R. & Pittet, M. J. Imaging in the era of molecular oncology. Nature 452, 580–589 (2008).
Giljohann, D. A. & Mirkin, C. A. Drivers of biodiagnostic development. Nature 462, 461–464 (2009).
Choi, H. S. et al. Design considerations for tumour-targeted nanoparticles. Nature Nanotech. 5, 42–47 (2010).
Jin, Y. & Gao, X. Plasmonic fluorescent quantum dots. Nature Nanotech. 4, 571–576 (2009).
Chen, Z. et al. Protein microarrays with carbon nanotubes as multicolor Raman labels. Nature Biotechnol. 26, 1285–1292 (2008).
Qian, X. et al. In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nature Biotechnol. 26, 83–90 (2008).
Lee, J. H. et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nature Med. 13, 95–99 (2007).
Gao, X., Cui, Y., Levenson, R. M., Chung, L. W. & Nie, S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nature Biotechnol. 22, 969–976 (2004).
Nam, J. M., Thaxton, C. S. & Mirkin, C. A. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science 301, 1884–1886 (2003).
Peer, D. et al. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotech. 2, 751–760 (2007).
Cho, K., Wang, X., Nie, S., Chen, Z. G. & Shin, D. M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 14, 1310–1316 (2008).
Akinc, A. et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nature Biotechnol. 26, 561–569 (2008).
Shaw, S. Y. et al. Perturbational profiling of nanomaterial biologic activity. Proc. Natl Acad. Sci. USA 105, 7387–7392 (2008).
Xing, Y. et al. Bioconjugated quantum dots for multiplexed and quantitative immunohistochemistry. Nature Protoc. 2, 1152–1165 (2007).
Devaraj, N. K., Upadhyay, R., Haun, J. B., Hilderbrand, S. A. & Weissleder, R. Fast and sensitive pretargeted labeling of cancer cells through a tetrazine/trans-cyclooctene cycloaddition. Angew. Chem. Int. Ed. 48, 7013–7016 (2009).
Devaraj, N. K., Weissleder, R. & Hilderbrand, S. A. Tetrazine-based cycloadditions: application to pretargeted live cell imaging. Bioconjug. Chem. 19, 2297–2299 (2008).
Blackman, M. L., Royzen, M. & Fox, J. M. Tetrazine ligation: fast bioconjugation based on inverse-electron-demand Diels–Alder reactivity. J. Am. Chem. Soc. 130, 13518–13519 (2008).
Lee, H., Sun, E., Ham, D. & Weissleder, R. Chip-NMR biosensor for detection and molecular analysis of cells. Nature Med. 14, 869–874 (2008).
Lee, H., Yoon, T. J., Figueiredo, J. L., Swirski, F. K. & Weissleder, R. Rapid detection and profiling of cancer cells in fine-needle aspirates. Proc. Natl Acad. Sci. USA 106, 12459–12464 (2009).
Hudis, C. A. Trastuzumab—mechanism of action and use in clinical practice. N. Engl. J. Med. 357, 39–51 (2007).
Hong, K. W. et al. A novel anti-EGFR monoclonal antibody inhibiting tumor cell growth by recognizing different epitopes from cetuximab. J. Biotechnol. 145, 84–91 (2010).
Troise, F., Cafaro, V., Giancola, C., D'Alessio, G. & De Lorenzo, C. Differential binding of human immunoagents and Herceptin to the ErbB2 receptor. FEBS J. 275, 4967–4979 (2008).
Green, N. M. Avidin and streptavidin. Methods Enzymol. 184, 51–67 (1990).
Chinol, M. et al. Biochemical modifications of avidin improve pharmacokinetics and biodistribution, and reduce immunogenicity. Br. J. Cancer 78, 189–197 (1998).
Nagrath, S. et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450, 1235–1239 (2007).
Maheswaran, S. et al. Detection of mutations in EGFR in circulating lung-cancer cells. N. Engl. J. Med. 359, 366–377 (2008).
Pantel, K., Brakenhoff, R. H. & Brandt, B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nature Rev. Cancer 8, 329–340 (2008).
Reynolds, F., O'Loughlin, T., Weissleder, R. & Josephson, L. Method of determining nanoparticle core weight. Anal. Chem. 77, 814–817 (2005).
Piran, U. & Riordan, W. J. Dissociation rate constant of the biotin–streptavidin complex. J. Immunol. Methods 133, 141–143 (1990).
Acknowledgements
The authors gratefully acknowledge N. Sergeyev for synthesizing CLIO and C. Wang for assistance with MALDI-TOF measurements. We especially thank G. Thurber, M. Pittet, F. Swirski and M. Nahrendorf for their many helpful suggestions. We also thank Y. Fisher-Jeffes for reviewing the manuscript. This work was funded in part by NCI grant P50CA86355 and RO1 EB004626.
Author information
Authors and Affiliations
Contributions
J.B.H. designed and performed the experiments, analysed the data and wrote the manuscript. N.K.D. and S.A.H. developed and synthesized the bioorthogonal chemistries. H.L. performed the magnetic resonance measurements. R.W. provided overall guidance, designed experiments, reviewed the data and wrote the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 891 kb)
Rights and permissions
About this article
Cite this article
Haun, J., Devaraj, N., Hilderbrand, S. et al. Bioorthogonal chemistry amplifies nanoparticle binding and enhances the sensitivity of cell detection. Nature Nanotech 5, 660–665 (2010). https://doi.org/10.1038/nnano.2010.148
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2010.148
This article is cited by
-
Exploration and functionalization of M1-macrophage extracellular vesicles for effective accumulation in glioblastoma and strong synergistic therapeutic effects
Signal Transduction and Targeted Therapy (2022)
-
Bioorthogonal chemistry
Nature Reviews Methods Primers (2021)
-
Molecular phenotyping of oxidative stress in diabetes mellitus with point-of-care NMR system
npj Aging and Mechanisms of Disease (2020)
-
Facile, productive, and cost-effective synthesis of a novel tetrazine-based iron oxide nanoparticle for targeted image contrast agents and nanomedicines
Journal of Nanoparticle Research (2018)
-
A fluorescent DNA based probe for Hg(II) based on thymine-Hg(II)-thymine interaction and enrichment via magnetized graphene oxide
Microchimica Acta (2018)