Abstract
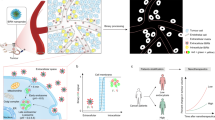
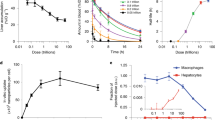
Nanoparticles have great potential as controllable drug delivery vehicles because of their size and modular functionality. Timing and location are important parameters when optimizing nanoparticles for delivery of chemotherapeutics. Here, we show that gold nanoparticles carrying either fluorescein or doxorubicin molecules move and localize differently in an in vitro three-dimensional model of tumour tissue, depending on whether the nanoparticles are positively or negatively charged. Fluorescence microscopy and mathematical modelling show that uptake, not diffusion, is the dominant mechanism in particle delivery. Our results indicate that positive particles may be more effective for drug delivery because they are taken up to a greater extent by proliferating cells. Negative particles, which diffuse more quickly, may perform better when delivering drugs deep into tissues. An understanding of how surface charge can control tissue penetration and drug release may overcome some of the current limitations in drug delivery.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hicks, K. O., Pruijn, F. B., Sturman, J. R., Denny, W. A. & Wilson, W. R. Multicellular resistance to tirapazamine is due to restricted extravascular transport: a pharmacokinetic/pharmacodynamic study in HT29 multicellular layer cultures. Cancer Res. 63, 5970–5977 (2003).
Lankelma, J. et al. Doxorubicin gradients in human breast cancer. Clin. Cancer Res. 5, 1703–1707 (1999).
Minchinton, A. I. & Tannock, I. F. Drug penetration in solid tumours. Nature Rev. Cancer 6, 583–592 (2006).
Jain, R. K. Transport of molecules, particles and cells in solid tumors. Annu. Rev. Biomed. Eng. 1, 241–263 (1999).
Primeau, A. J., Rendon, A., Hedley, D., Lilge, L. & Tannock, I. F. The distribution of the anticancer drug doxorubicin in relation to blood vessels in solid tumors. Clin. Cancer Res. 11, 8782–8788 (2005).
Vaupel, P., Kallinowski, F. & Okunieff, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 49, 6449–6465 (1989).
Tredan, O., Galmarini, C. M., Patel, K. & Tannock, I. F. Drug resistance and the solid tumor microenvironment. J. Natl Cancer Inst. 99, 1441–1454 (2007).
Grantab, R., Sivananthan, S. & Tannock, I. F. The penetration of anticancer drugs through tumor tissue as a function of cellular adhesion and packing density of tumor cells. Cancer Res. 66, 1033–1039 (2006).
Goldacre, R. J. & Sylven, B. On the access of blood-borne dyes to various tumour regions. Br. J. Cancer 16, 306–322 (1962).
You, C. C., Verma, A. & Rotello, V. M. Engineering the nanoparticle–biomacromolecule interface. Soft Matter 2, 190–204 (2006).
You, C. C., De, M., Han, G. & Rotello, V. M. Tunable inhibition and denaturation of alpha-chymotrypsin with amino acid-functionalized gold nanoparticles. J. Am. Chem. Soc. 127, 12873–12881 (2005).
Goodman, C. M. et al. DNA-binding by functionalized gold nanoparticles: mechanism and structural requirements. Chem. Biol. Drug. Des. 67, 297–304 (2006).
McIntosh, C. M. et al. Inhibition of DNA transcription using cationic mixed monolayer protected gold clusters. J. Am. Chem. Soc. 123, 7626–7629 (2001).
You, C. C., De, M., Han, G. & Rotello, V. M. Tunable inhibition and denaturation of alpha-chymotrypsin with amino acid-functionalized gold nanoparticles. J. Am. Chem. Soc. 127, 12873–12881 (2005).
Thomas, M. & Klibanov, A. M. Conjugation to gold nanoparticles enhances polyethylenimine's transfer of plasmid DNA into mammalian cells. Proc. Natl Acad. Sci. USA 100, 9138–9143 (2003).
Rosi, N. L. et al. Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. Science 312, 1027–1030 (2006).
Jain, P. K., El-Sayed, I. H. & El-Sayed, M. A. Au nanoparticles target cancer. Nano Today 2, 18–29 (2007).
Chithrani, B. D. & Chan, W. C. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 7, 1542–1550 (2007).
Kasinskas, R. W. & Forbes, N. S. Salmonella typhimurium specifically chemotax and proliferate in heterogeneous tumor tissue in vitro. Biotechnol. Bioeng. 94, 710–721 (2006).
Kim, B. J. & Forbes, N. S. Flux analysis shows that hypoxia-inducible-factor-1-alpha minimally affects intracellular metabolism in tumor spheroids. Biotechnol. Bioeng. 96, 1167–1182 (2007).
Freyer, J. P. & Sutherland, R. M. Selective dissociation and characterization of cells from different regions of multicell tumor spheroids. Cancer Res. 40, 3956–3965 (1980).
Kasinskas, R. W. & Forbes, N. S. Salmonella typhimurium lacking ribose chemoreceptors localize in tumor quiescence and induce apoptosis. Cancer Res. 67, 3201–3209 (2007).
Sutherland, R. M. Cell and environment interactions in tumor microregions—the multicell spheroid model. Science 240, 177–184 (1988).
Kim, B. J. & Forbes, N. S. Single-cell analysis demonstrates how nutrient deprivation creates apoptotic and quiescent cell populations in tumor cylindroids. Biotechnol. Bioeng. 101, 797–810 (2008).
Helmlinger, G., Yuan, F., Dellian, M. & Jain, R. K. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nature Med. 3, 177–182 (1997).
De, M., You, C. C., Srivastava, S. & Rotello, V. M. Biomimetic interactions of proteins with functionalized nanoparticies: a thermodynamic study. J. Am. Chem. Soc. 129, 10747–10753 (2007).
Chompoosor, A., Han, G. & Rotello, V. M. Charge dependence of ligand release and monolayer stability of gold nanoparticles by biogenic thiols. Bioconjug. Chem. 19, 1342–1345 (2008).
Hong, R. et al. Glutathione-mediated delivery and release using monolayer protected nanoparticle carriers. J. Am. Chem. Soc. 128, 1078–1079 (2006).
Jones, D. P. et al. Glutathione measurement in human plasma evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin. Chim. Acta 275, 175–184 (1998).
Hassan, S. S. M. & Rechnitz, G. A. Determination of glutathione and glutathione-reductase with a silver sulfide membrane-electrode. Anal. Chem. 54, 1972–1976 (1982).
Baldwin, A. L., Wu, N. Z. & Stein, D. L. Endothelial surface-charge of intestinal mucosal capillaries and its modulation by dextran. Microvasc. Res. 42, 160–178 (1991).
Ghitescu, L. & Fixman, A. Surface-charge distribution on the endothelial-cell of liver sinusoids. J. Cell Biol. 99, 639–647 (1984).
Chen, A. M. et al. Oligodeoxynucleotide nanostructure formation in the presence of polypropyleneimine dendrimers and their uptake in breast cancer cells. Nanotechnology 17, 5449–5460 (2006).
Holzapfel, V., Musyanovych, A., Landfester, K., Lorenz, M. R. & Mailander, V. Preparation of fluorescent carboxyl and amino functionalized polystyrene particles by miniemulsion polymerization as markers for cells. Macromol. Chem. Phys. 206, 2440–2449 (2005).
Mislick, K. A. & Baldeschwieler, J. D. Evidence for the role of proteoglycans in cation-mediated gene transfer. Proc. Natl Acad. Sci. USA 93, 12349–12354 (1996).
Tauskela, J. S. et al. Evaluation of glutathione-sensitive fluorescent dyes in cortical culture. Glia 30, 329–341 (2000).
Orwar, O., Fishman, H. A., Ziv, N. E., Scheller, R. H. & Zare, R. N. Use of 2,3-naphthalenedicarboxaldehyde derivatization for single-cell analysis of glutathione by capillary electrophoresis and histochemical-localization ion by fluorescence microscopy. Anal. Chem. 67, 4261–4268 (1995).
Davies, C. D., Berk, D. A., Pluen, A. & Jain, R. K. Comparison of IgG diffusion and extracellular matrix composition in rhabdomyosarcomas grown in mice versus in vitro as spheroids reveals the role of host stromal cells. Br. J. Cancer 86, 1639–1644 (2002).
Pluen, A. et al. Role of tumor–host interactions in interstitial diffusion of macromolecules: cranial vs. subcutaneous tumors. Proc. Natl Acad. Sci. USA 98, 4628–4633 (2001).
Ramanujan, S. et al. Diffusion and convection in collagen gels: implications for transport in the tumor interstitium. Biophys. J. 83, 1650–1660 (2002).
Kleinman, H. K. et al. Isolation and characterization of type-IV procollagen, laminin, and heparan-sulfate proteoglycan from the Ehs sarcoma. Biochemistry (Mosc) 21, 6188–6193 (1982).
Boucher, Y., Baxter, L. T. & Jain, R. K. Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer Res. 50, 4478–4484 (1990).
Jain, R. K. Transport of molecules in the tumor interstitium: a review. Cancer Res. 47, 3039–3051 (1987).
Young, J. S., Lumsden, C. E. & Stalker, A. L. The significance of the tissue pressure of normal testicular and of neoplastic (Brown–Pearce carcinoma) tissue in the rabbit. J. Pathol. Bacteriol. 62, 313–333 (1950).
You, C. C., De, M. & Rotello, V. M. Contrasting effects of exterior and interior hydrophobic moieties in the complexation of amino acid functionalized gold clusters with alpha-chymotrypsin. Org. Lett. 7, 5685–5688 (2005).
Paciotti, G. F. et al. Colloidal gold: a novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 11, 169–183 (2004).
Acknowledgements
The authors gratefully acknowledge financial support from the National Institutes of Health (grant nos 1R21CA112335-01A and 1R01CA120825-01A1) and the National Science Foundation (grant no. DMI-0531171).
Author information
Authors and Affiliations
Contributions
N.F. and V.R. conceived and designed the experiments. B.K., G.H., B.T. and C.K. performed the experiments. G.H. synthesized the FITC nanoparticles. C.K. synthesized the DOX nanoparticles. B.K. performed all cell and cylindroid experiments with FITC nanoparticles and wrote all mathematical models. B.T. performed all cylindroid and cell experiments with DOX nanoparticles. B.K., B.T. and N.F. analysed the data. V.R. contributed materials. B.K. and N.F. co-wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 838 kb)
Rights and permissions
About this article
Cite this article
Kim, B., Han, G., Toley, B. et al. Tuning payload delivery in tumour cylindroids using gold nanoparticles. Nature Nanotech 5, 465–472 (2010). https://doi.org/10.1038/nnano.2010.58
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2010.58
This article is cited by
-
Immobilization of azide-functionalized proteins to micro- and nanoparticles directly from cell lysate
Microchimica Acta (2024)
-
Biological Interaction and Imaging of Ultrasmall Gold Nanoparticles
Nano-Micro Letters (2024)
-
Numerical simulation for a Casson nanofluid over an inclined vessel surrounded by hot tissue at the microscale
SN Applied Sciences (2023)
-
A mathematical modeling approach toward magnetic fluid hyperthermia of cancer and unfolding heating mechanism
Journal of Thermal Analysis and Calorimetry (2021)
-
Targeted crystallization of mixed-charge nanoparticles in lysosomes induces selective death of cancer cells
Nature Nanotechnology (2020)