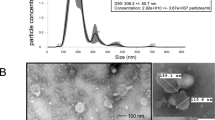
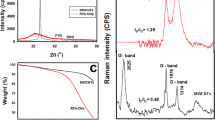
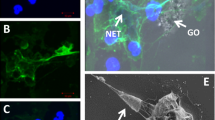
Abstract
We have shown previously that single-walled carbon nanotubes can be catalytically biodegraded over several weeks by the plant-derived enzyme, horseradish peroxidase1. However, whether peroxidase intermediates generated inside human cells or biofluids are involved in the biodegradation of carbon nanotubes has not been explored. Here, we show that hypochlorite and reactive radical intermediates of the human neutrophil enzyme myeloperoxidase catalyse the biodegradation of single-walled carbon nanotubes in vitro, in neutrophils and to a lesser degree in macrophages. Molecular modelling suggests that interactions of basic amino acids of the enzyme with the carboxyls on the carbon nanotubes position the nanotubes near the catalytic site. Importantly, the biodegraded nanotubes do not generate an inflammatory response when aspirated into the lungs of mice. Our findings suggest that the extent to which carbon nanotubes are biodegraded may be a major determinant of the scale and severity of the associated inflammatory responses in exposed individuals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Allen, B. L. et al. Biodegradation of single-walled carbon nanotubes through enzymatic catalysis. Nano Lett. 8, 3899–3903 (2008).
Maynard, A. D. et al. Safe handling of nanotechnology. Nature 444, 267–269 (2006).
Shvedova, A. A. et al. Mechanisms of pulmonary toxicity and medical applications of carbon nanotubes: two faces of Janus? Pharmacol. Ther. 121, 192–204 (2009).
Jia, G. et al. Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube and fullerene. Environ. Sci. Technol. 39, 1378–1383 (2005).
Kagan, V. E. et al. Direct and indirect effects of single walled carbon nanotubes on RAW 264.7 macrophages: role of iron. Toxicol. Lett. 165, 88–100 (2006).
Kisin, E. R. et al. Single-walled carbon nanotubes: geno- and cytotoxic effects in lung fibroblast V79 cells. J. Toxicol. Environ. Health. A 70, 2071–2079 (2007).
Shvedova, A. A. et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 289, L698–L708 (2005).
Shvedova, A. A. et al. Inhalation vs. aspiration of single-walled carbon nanotubes in C57BL/6 mice: inflammation, fibrosis, oxidative stress and mutagenesis. Am. J. Physiol. Lung Cell. Mol. Physiol. 295, L552–L565 (2008).
Muller, J. et al. Structural defects play a major role in the acute lung toxicity of multiwall carbon nanotubes: toxicological aspects. Chem. Res. Toxicol. 21, 1698–1705 (2008).
Poland, C. A. et al. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nature Nanotech. 3, 423–428 (2008).
Wei, Z., Kondratenko, M., Dao, L. H. & Perepichka, D. F. Rectifying diodes from asymmetrically functionalized single-wall carbon nanotubes. J. Am. Chem. Soc. 128, 3134–3135 (2006).
Yoon, S. M. et al. Selective oxidation on metallic carbon nanotubes by halogen oxoanions. J. Am. Chem. Soc. 130, 2610–2616 (2008).
Wu, C.-H. Studies of the equilibrium and thermodynamics of the absorption of Cu2+ onto as-produced and modified carbon nanotubes. J. Colloid Interface Sci. 311, 338–346 (2007).
Hampton, M. B., Kettle, A. J. & Winterbourn, C. C. Inside the neutrophil phagosome: oxidants, myeloperoxidase and bacterial killing. Blood 92, 3007–3017 (1998).
Sutherland, K., Mahoney, J. R., II, Coury, A. J. & Eaton, J. W. Degradation of biomaterials by phagocyte-derived oxidants. J. Clin. Invest. 92, 2360–2367 (1993).
Kuznetsova, A. et al. Oxygen-containing functional groups on single-wall carbon nanotubes: NEXAFS and vibrational spectroscopic studies. J. Am. Chem. Soc. 123, 10699–10704 (2001).
Panasenko, O. M. et al. Generation of free radicals during decomposition of hydroperoxide in the presence of myeloperoxidase or activated neutrophils. Biochemistry (Mosc) 70, 998–1004 (2005).
Kearns, S. & Dawson, R., Jr. Cytoprotective effect of taurine against hypochlorous acid toxicity to PC12 cells. Adv. Exp. Med. Biol. 483, 563–570 (2000).
Lessig, J. et al. Myeloperoxidase binds to non-vital spermatozoa on phosphatidylserine epitopes. Apoptosis 12, 1803–1812 (2007).
Olivier, M. L. & Paul, R. O. EPR spin-trapping of a myeloperoxidase protein radical. Biochem. Biophys. Res. Commun. 1, 199–202 (2000).
Kam, N. & Dai, H. Single walled carbon nantoubes for transport and delivery of biological cargos. Phys. Stat. Sol. 243, 3561–3566 (2006).
Zipfel, M., Carmine, T. C., Gerber, C., Niethammer, D. & Bruchelt, G. Evidence for the activation of myeloperoxidase by f-Meth-Leu-Phe prior to its release from neutrophil granulocytes. Biochem. Biophys. Res. Commun. 232, 209–212 (1997).
McKenzie, S. E. & Schreiber, A. D. Fc gamma receptors in phagocytes. Curr. Opin. Hematol. 5, 16–21 (1998).
Kettle, A. J., Gedye, C. A. & Winterbourn, C. C. Mechanism of inactivation of myeloperoxidase by 4-aminobenzoic acid hydrazide. Biochem. J. 321, 503–508 (1997).
Touyz, R. M. Apocynin, NADPH oxidase and vascular cells: a complex matter. Hypertension 51, 172–174 (2008).
Batrakova, E. V. et al. A macrophage–nanozyme delivery system for Parkinson's disease. Bioconjug. Chem. 18, 1498–1506 (2007).
Dou, H. et al. Development of a macrophage-based nanoparticle platform for antiretroviral drug delivery. Blood 108, 2827–2835 (2006).
Konduru, N. V. et al. Phosphatidylserine targets single-walled carbon nanotubes to professional phagocytes in vitro and in vivo. PLoS One 4, e4398 (2009).
DeLano, W. L. The PyMOL Molecular Graphics System (DeLano Scientific, 2002).
Yanamala, N., Tirupula, K. C. & Klein-Seetharaman, J. Preferential binding of allosteric modulators to active and inactive conformational states of metabotropic glutamate receptors. BMC Bioinformatics 9(Suppl 1), S16 (2008).
Acknowledgements
This work was supported by grants from National Institute for Occupational Safety and Health (NIOSH) OH008282, National Institutes of Health HL70755, HL094488, U19AI068021, National Library of Medicine LM007994-05, National Occupational Research Agenda (NORA) 927000Y, 927Z1LU, Nanotechnology Research Center (NTRC) 927ZJHF, National Science Foundation (NSF) CAREER 0449117, Air Force Office of Scientific Research (AFOSR) FA9550-09-1-0478, 7th Framework Program of the European Commission (EC-FP7-NANOMMUNE-214281) and by the Science Foundation of Ireland, Strategic Research Cluster (SRC) BioNanointeract and Centre for Research on Adaptive Nanostructures and Nanodevices (CRANN), Higher Education Authority (HEA) and Programme for Research in Third-Level Institutions (PRTLI). The authors would like to thank Marcel Bruchez for assistance with dynamic light scattering experiments.
Author information
Authors and Affiliations
Contributions
V.E.K., N.V.K., B.F. and A.S. designed the experiments, analysed the data and wrote the manuscript. W.F., J.S. and N.V.K. performed the neutrophil-based experiments. B.L.A. and N.V.K. participated in spectroscopic studies. I.I.V. and A.K. performed ESR measurements and gel electrophoresis. E.R.K., A.R.M. and A.S. designed and performed the animal experiments and evaluated the data. N.Y. and J.K.S. performed the molecular modelling studies. J.F. and D.S. carried out the electron microscopic studies. P.G. synthesized fluorescence labelled nanotubes. J.C. and Y.V. carried out the Raman microscopic studies. N.A.B. and Y.Y.T. performed the mass spectrometric analysis. All co-authors discussed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1954 kb)
Rights and permissions
About this article
Cite this article
Kagan, V., Konduru, N., Feng, W. et al. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nature Nanotech 5, 354–359 (2010). https://doi.org/10.1038/nnano.2010.44
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2010.44
This article is cited by
-
Gobind’s last graduate student
Biophysical Reviews (2023)
-
Detection of HOCl-driven degradation of the pericardium scaffolds by label-free multiphoton fluorescence lifetime imaging
Scientific Reports (2022)
-
Unifying structural descriptors for biological and bioinspired nanoscale complexes
Nature Computational Science (2022)
-
MXene in the lens of biomedical engineering: synthesis, applications and future outlook
BioMedical Engineering OnLine (2021)
-
The Comparative Study of Gelatin/CNT-contained Mg-Ca-P Bone Cement with the Plain and CNT-reinforced Ones
Journal of Bionic Engineering (2021)