Abstract
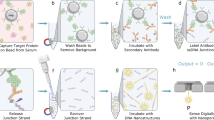
Label-free nanosensors can detect disease markers to provide point-of-care diagnosis that is low-cost, rapid, specific and sensitive1,2,3,4,5,6,7,8,9,10,11,12,13. However, detecting these biomarkers in physiological fluid samples is difficult because of problems such as biofouling and non-specific binding, and the resulting need to use purified buffers greatly reduces the clinical relevance of these sensors. Here, we overcome this limitation by using distinct components within the sensor to perform purification and detection. A microfluidic purification chip simultaneously captures multiple biomarkers from blood samples and releases them, after washing, into purified buffer for sensing by a silicon nanoribbon detector. This two-stage approach isolates the detector from the complex environment of whole blood, and reduces its minimum required sensitivity by effectively pre-concentrating the biomarkers. We show specific and quantitative detection of two model cancer antigens from a 10 µl sample of whole blood in less than 20 min. This study marks the first use of label-free nanosensors with physiological solutions, positioning this technology for rapid translation to clinical settings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Sander, C. Genomic medicine and the future of health care. Science 287, 1977–1978 (2000).
Jemal, A. et al. Cancer statistics 2008. CA Cancer J. Clin. 58, 71–96 (2008).
Etzioni, R. et al. The case for early detection. Nature Rev. Cancer 3, 243–252 (2003).
Liang, S. & Chan, D. W. Enzymes and related proteins as cancer biomarkers: a proteomic approach. Clin. Chim. Acta 381, 93–97 (2007).
Fan, R. et al. Integrated barcode chips for rapid, multiplexed analysis of proteins in microliter quantities of blood. Nature Biotechnol. 26, 1373–1378 (2008).
Zheng, G., Patolsky, F., Cui, Y., Wang, W. U. & Lieber, C. M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nature Biotechnol. 23, 1294–1301 (2005).
Cui, Y., Wei, Q., Park, H. & Lieber, C. M. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 293, 1289–1292 (2001).
Jain, K. K. Nanotechnology in clinical laboratory diagnostics. Clin. Chim. Acta 358, 37–54 (2005).
Burg, Thomas T. P. et al. Weighing of biomolecules, single cells and single nanoparticles in fluid. Nature 446, 1066–1069 (2007).
Kim, A. et al. Ultrasensitive, label-free and real-time immunodetection using silicon field-effect transistors. Appl. Phys. Lett. 91, 103901 (2007).
Stern, E. et al. Label-free immunodetection with CMOS-compatible semiconducting nanowires. Nature 445, 519–522 (2007).
Stern, E., Vacic, A. & Reed, M. A. Semiconducting nanowire field-effect transistor biomolecular sensors. IEEE Trans. Electron. Dev. 55, 3119–3130 (2008).
Bunimovich, Y. L. et al. Quantitative real-time measurements of DNA hybridization with alkylated nonoxidized silicon nanowires in electrolyte solution. J. Am. Chem. Soc. 128, 16323–16331 (2006).
Nagrath, S. et al. Isolation of rare circulating tumor cells in cancer patients by microchip technology. Nature 450, 1235–1239 (2007).
Gupta, A. K. et al. Anomalous resonance in a nanomechanical biosensor. Proc. Natl Acad. Sci. USA 103, 13362–13367 (2006).
Stern, E. et al. Importance of the Debye screening length on nanowire field effect transistor sensors. Nano Lett. 7, 3405–3409 (2007).
Zhou, H., Ranish, J. A., Watts, J. D. & Aebersold, R. Quantitative proteome analysis by solid-phase isotope tagging and mass spectrometry. Nature Biotechnol. 20, 512–515 (2002).
Templin, M. F., Stoll, D., Bachmann, J. & Joos, T. O. Protein microarrays and multiplexed sandwich immunoassays: what beats the beads? Comb. Chem. High Through. Screen 7, 223–229 (2004).
Hermanson, G. T. Bioconjugate Techniques (Elsevier, 1996).
Bai, X., Kim, S., Li, Z., Turro, N. J. & Ju, J. Design and synthesis of a photocleavable biotinylated nucleotide for DNA analysis by mass spectrometry. Nucleic Acids Res. 32, 535–541 (2004).
Handwerger, R. G. & Diamond, S. L. Biotinylated photocleavable polyethylenimine: capture and triggered release of nucleic acids from solid supports. Bioconjug. Chem. 18, 717–723 (2007).
Senter, P. D. et al. Novel photocleavable protein crosslinking reagents and their use in the preparation of antibody–toxin conjugates. Photochem. Photobiol. 42, 231–237 (1985).
Olejnik, J. et al. Photocleavable biotin derivatives—a versatile approach for the isolation of biomolecules. Proc. Natl Acad. Sci. USA 92, 7590–7594 (1995).
Vickers, A. J., Savage, C., O'Brien, M. F. & Lilja, H. Systematic review of pretreatment prostate-specific antigen velocity and doubling time as predictors for prostate cancer. J. Clin. Oncol. 27, 398–403 (2009).
Shariat, S. F., Scardino, P. T. & Lilja, H. Screening for prostate cancer: an update. Can. J. Urol. 15, 4363–4374 (2008).
Rubach, M., Szymendera, J. J., Kaminska, J. & Kowalska, M. Serum CA 15.3, CEA and ESR patterns in breast cancer. Int. J. Biol. Markers 12, 168–173 (1997).
Uehara, M. et al. Long-term prognostic study of carcinoembryonic antigen (CEA) and carbohydrate antigen 15-3 (CA 15-3) in breast cancer. Int. J. Clin. Oncol. 13, 447–451 (2008).
Elfstrom, N., Karlstrom, A. E. & Linnros, J. Silicon nanoribbons for electrical detection of biomolecules. Nano Lett. 8, 945–949 (2008).
Cantor, C. R. & Schimmel, P. R. Biophysical Chemistry: Part III: The Behavior of Biological Macromolecules (Freeman, 1980).
Homola, J. Present and future of surface plasmon resonance biosensors. Anal. Bioanal. Chem. 377, 528–539 (2003).
Acknowledgements
The authors would like to thank J. Straight for many helpful discussions, M. Look and J. Bertram for blood samples, M. Power for device processing assistance, M. Saltzman for departmental support, and D. Stern and K. Milnamow for critical reading of the manuscript. The work was supported in part by the National Institute of Health (NIH) through grant no. R01EB008260 (M.A.R. and T.M.F.), Canadian Institute for Advanced Research (CIfAR), and Army Research Office (ARO) (W911NF-08-1-0365). This work was performed in part at the Cornell Nanoscale Science and Technology Facility, a member of the National Nanotechnology Infrastructure Network that is supported by the National Science Foundation (NSF), and at the Yale Institute for Nanoscience and Quantum Engineering. This paper is dedicated to the memory of Alan R. Stern.
Author information
Authors and Affiliations
Contributions
E.S. designed the MPC and performed all MPC experiments. E.S. and B.R.I. designed the MPC fabrication and performed MPC processing. E.S., A.V. and M.A.R. designed the nanosensor fabrication process and E.S., A.V. and B.R.I. performed nanosensor processing. D.J.M. assisted with MPC and nanosensor experimental design, and data analysis. E.S., A.V., N.K.R. and J.M.C. performed the sensing measurements. E.S., J.M.C. and J.P. prepared and analysed the protein samples. E.S., M.A.R. and T.M.F. wrote the manuscript and edited it, with contributions from all authors.
Corresponding authors
Supplementary information
Supplementary information
Supplementary information (PDF 2005 kb)
Rights and permissions
About this article
Cite this article
Stern, E., Vacic, A., Rajan, N. et al. Label-free biomarker detection from whole blood. Nature Nanotech 5, 138–142 (2010). https://doi.org/10.1038/nnano.2009.353
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2009.353
This article is cited by
-
Ultrasensitive rapid cytokine sensors based on asymmetric geometry two-dimensional MoS2 diodes
Nature Communications (2022)
-
A controllable fabrication improved silicon nanowire array sensor on (111) SOI for accurate bio-analysis application
Nano Research (2022)
-
Nanomaterial-Based Biosensors using Field-Effect Transistors: A Review
Journal of Electronic Materials (2022)
-
Cancer nanotechnology: current status and perspectives
Nano Convergence (2021)
-
Distance-based quantification of miRNA-21 by the coffee-ring effect using paper devices
Microchimica Acta (2020)