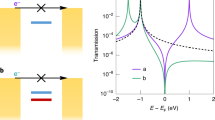
Abstract
If individual molecules are to be used as building blocks for electronic devices, it will be essential to understand charge transport at the level of single molecules. Most existing experiments rely on the synthesis of functional rod-like molecules with chemical linker groups at both ends to provide strong, covalent anchoring to the source and drain contacts. This approach has proved very successful, providing quantitative measures of single-molecule conductance, and demonstrating rectification and switching at the single-molecule level. However, the influence of intermolecular interactions on the formation and operation of molecular junctions has been overlooked. Here we report the use of oligo-phenylene ethynylene molecules as a model system, and establish that molecular junctions can still form when one of the chemical linker groups is displaced or even fully removed. Our results demonstrate that aromatic π−π coupling between adjacent molecules is efficient enough to allow for the controlled formation of molecular bridges between nearby electrodes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ratner, M. A. & Aviram, A. Molecular rectifiers. Chem. Phys. Lett. 29, 277–283 (1974).
Joachim, C., Gimzewski, J. K. & Aviram, A. Electronics using hybrid-molecular and mono-molecular devices. Nature 408, 541–548 (2000).
Nitzan, A. & Ratner, M. A. Electron transport in molecular wire junctions. Science 300, 1384–1389 (2003).
Joachim, C. & Ratner, M. A. Molecular electronics special feature: Molecular electronics: some views on transport junctions and beyond. Proc. Natl Acad. Sci. USA 102, 8801–8808 (2005).
Selzer, Y. & Allara, D. L. Single-molecule electrical junctions. Ann. Rev. Phys. Chem. 57, 593–623 (2006).
Tao, N. J. Electron transport in molecular junctions. Nature Nanotech. 1, 173–181 (2006).
Lindsay, S. M. & Ratner, M. A. Molecular transport junctions: Clearing mists. Adv. Mater. 19, 23–31 (2007).
Weibel, N., Grunder, S. & Mayor, M. Functional molecules in electronic circuits. Org. Biomol. Chem. 5, 2343–2353 (2007).
Mantooth, B. & Weiss, P. Fabrication, assembly, and characterization of molecular electronic components. Proc. IEEE 91, 1785–1802 (2003).
Reed, M. A., Zhou, C., Müller, C. J., Burgin, T. P. & Tour, J. M. Conductance of a molecular junction. Science 278, 252–254 (1997).
Kergueris, C. et al. Electron transport through a metal–molecule–metal junction. Phys. Rev. B 59, 12505–12513 (1999).
Smit, R. H. M. et al. Measurement of the conductance of a hydrogen molecule. Nature 419, 906–909 (2002).
Reichert, J. et al. Driving current through single organic molecules. Phys. Rev. Lett. 88, 176804 (2002).
Csonka, S. et al. Fractional conductance in hydrogen-embedded gold nanowires. Phys. Rev. Lett. 90, 116803 (2003).
Böhler, T., Grebing, J., Mayer-Gindner, A., von Löhneysen, H. & Scheer, E. Mechanically controllable break-junctions for use as electrodes for molecular electronics. Nanotechnology 15, S465–S471 (2004).
Champagne, A., Pasupathy, A. & Ralph, D. Mechanically adjustable and electrically gated single-molecule transistors. Nano Lett. 5, 305–308 (2005).
González, M. T. et al. Electrical conductance of molecular junctions by a robust statistical analysis. Nano Lett. 6, 2238–2242 (2006).
Lörtscher, E., Ciszek, J., Tour, J. & Riel, H. Reversible and controllable switching of a single-molecule junction. Small 2, 973–977 (2006).
Agrait, N., Yeyati, A. L. & van Ruitenbeek, J. M. Quantum properties of atomic-sized conductors. Phys. Rep. 377, 81–279 (2003).
Xu, B., Xiao, X. & Tao, N. Measurements of single-molecule electromechanical properties. J. Am. Chem. Soc. 125, 16164–16165 (2003).
Huang, Z., Chen, F., Bennett, P. & Tao, N. Single molecule junctions formed via Au-thiol contact: Stability and breakdown mechanism. J. Am. Chem. Soc. 129, 13225–13231 (2007).
James, D. K. & Tour, J. M. Molecular wires. Top. Curr. Chem. 257, 33–62 (2005).
Weiss, E. A., Wasielewski, M. R. & Ratner, M. A. Molecules as wires: Molecule-assisted movement of charge and energy. Top. Curr. Chem. 257, 103–133 (2005).
Grüter, L., González, M. T., Huber, R., Calame, M. & Schönenberger, C. Electrical conductance of atomic contacts in a liquid environment. Small 1, 1067–1070 (2005).
Grüter, L. et al. Resonant tunnelling through a C60 molecular junction in a liquid environment. Nanotechnology 16, 2143–2148 (2005).
Huber, R. et al. Electrical conductance of conjugated oligomers at the single molecule level. J. Am. Chem. Soc. 130, 1080–1084 (2008).
Venkataraman, L. et al. Single-molecule circuits with well-defined molecular conductance. Nano Lett. 6, 458–462 (2006).
Li, X.-L. et al. Thermally activated electron transport in single redox molecules. J. Am. Chem. Soc. 129, 11535–11542 (2007).
Kim, K., Tarakeshwar, P. & Lee, J. Molecular clusters of π-systems: Theoretical studies of structures, spectra, and origin of interaction energies. Chem. Rev. 100, 4145–4186 (2000).
Watson, M., Fechtenkotter, A. & Müllen, K. Big is beautiful – “aromaticity” revisited from the viewpoint of macromolecular and supramolecular benzene chemistry. Chem. Rev. 101, 1267–1300 (2001).
Hoeben, F., Jonkheijm, P., Meijer, E. & Schenning, A. About supramolecular assemblies of π-conjugated systems. Chem. Rev. 105, 1491–1546 (2005).
Taylor, J., Brandbyge, M. & Stokbro, K. Conductance switching in a molecular device: The role of side groups and intermolecular interactions. Phys. Rev. B 68, 121101 (2003).
Blum, A. et al. Charge transport and scaling in molecular wires. J. Phys. Chem. B 108, 18124–18128 (2004).
Selzer, Y. et al. Effect of local environment on molecular conduction: Isolated molecule versus self-assembled monolayer. Nano Lett. 5, 61–65 (2005).
Galperin, M., Ratner, M. A. & Nitzan, A. Molecular transport junctions: vibrational effects. J. Phys. Condens. Matter 19, 103201 (2007).
Hunter, C. A. & Sanders, J. K. M. The nature of π–π interactions. J. Am. Chem. Soc. 112, 5525–5534 (1990).
Seminario, J., Zacarias, A. & Tour, J. Theoretical interpretation of conductivity measurements of a thiotolane sandwich. A molecular scale electronic controller. J. Am. Chem. Soc. 120, 3970–3974 (1998).
Levitus, M. et al. Steps to demarcate the effects of chromophore aggregation and planarization in poly(phenyleneethynylene)s. 1. Rotationally interrupted conjugation in the excited states of 1,4-bis(phenylethynyl)benzene. J. Am. Chem. Soc. 123, 4259–4265 (2001).
Okuyama, K., Hasegawa, T., Ito, M. & Mikami, N. Electronic-spectra of tolane in a supersonic free jet – large-amplitude torsional motion. J. Phys. Chem. 88, 1711–1716 (1984).
Miteva, T., Palmer, L., Kloppenburg, L., Neher, D. & Bunz, U. Interplay of thermochromicity and liquid crystalline behavior in poly(p-phenyleneethynylene)s: π–π interactions or planarization of the conjugated backbone? Macromolecules 33, 652–654 (2000).
Kim, J. & Swager, T. M. Control of conformational and interpolymer effects in conjugated polymers. Nature 411, 1030–1034 (2001).
Li, H., Powell, D. R., Firman, T. K. & West, R. Structures and photophysical properties of model compounds for arylethynylene disilylene polymers. Macromolecules 31, 1093–1098 (1998).
Wang, C., Batsanov, A., Bryce, M. & Sage, I. Nanoscale aryleneethynylene molecular wires with reversible fluorenone electrochemistry for self-assembly onto metal surfaces. Org. Lett. 6, 2181–2184 (2004).
Seferos, D. S., Trammell, S. A., Bazan, G. C. & Kushmerick, J. G. Probing π-coupling in molecular junctions. Proc. Natl Acad. Sci. USA 102, 8821–8825 (2005).
Creager, S. et al. Electron transfer at electrodes through conjugated “molecular wire” bridges. J. Am. Chem. Soc. 121, 1059–1064 (1999).
Schlicke, B., Belser, P., De Cola, L., Sabbioni, E. & Balzani, V. Photonic wires of nanometric dimensions. Electronic energy transfer in rigid rodlike Ru(bpy)32+-(ph)n-os(bpy)32+ compounds (ph = 1,4-phenylene; n = 3, 5, 7). J. Am. Chem. Soc. 121, 4207–4214 (1999).
Atienza, C. et al. Tuning electron transfer through p-phenyleneethynylene molecular wires. Chem. Commun. 30, 3202–3204 (2006).
Magoga, M. & Joachim, C. Conductance and transparence of long molecular wires. Phys. Rev. B 56, 4722–4729 (1997).
Tomfohr, J. K. & Sankey, O. F. Simple estimates of the electron transport properties of molecules. Phys. Status Solidi 233, 59–69 (2002).
Acknowledgements
This work was financed by the Swiss National Science Foundation and the National Center of Competence in Research ‘Nanoscale Science’. We also acknowledge the GEBERT RU¨F STIFTUNG for financial support. M.T.G. acknowledges the ‘Ministerio de Educación y Ciencia’ and the Freiwillige Akademische Gesellschaft for financial support.
Author information
Authors and Affiliations
Contributions
S.W., R.H. and M.T.G. carried out the experiments and conducted the analysis; S.G. synthesized the molecules; M.C., M.M. and C.S. designed the experiment, initiated the collaboration and supported the project in discussions; and S.W., C.S. and M.C. wrote the manuscript.
Corresponding authors
Supplementary information
Rights and permissions
About this article
Cite this article
Wu, S., González, M., Huber, R. et al. Molecular junctions based on aromatic coupling. Nature Nanotech 3, 569–574 (2008). https://doi.org/10.1038/nnano.2008.237
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2008.237
This article is cited by
-
L-Histidine-based computation devices
Pramana (2023)
-
σ–σ Stacked supramolecular junctions
Nature Chemistry (2022)
-
From molecular to supramolecular electronics
Nature Reviews Materials (2021)
-
In-situ formation of one-dimensional coordination polymers in molecular junctions
Nature Communications (2019)
-
Atomically defined angstrom-scale all-carbon junctions
Nature Communications (2019)