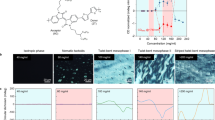
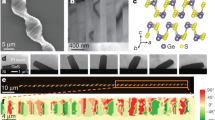
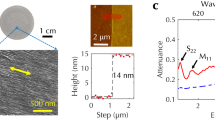
Abstract
Manipulating the morphology of inorganic nanostructures, such as their chirality and branching structure, has been actively pursued as a means of controlling their electrical, optical and mechanical properties. Notable examples of chiral inorganic nanostructures include carbon nanotubes1,2, gold multishell nanowires3, mesoporous nanowires4,5 and helical nanowires6,7,8. Branched nanostructures9,10,11,12,13,14,15,16 have also been studied and been shown to have interesting properties for energy harvesting17 and nanoelectronics18. Combining both chiral and branching motifs into nanostructures might provide new materials properties. Here we show a chiral branched PbSe nanowire structure, which is formed by a vapour–liquid–solid branching from a central nanowire with an axial screw dislocation. The chirality is caused by the elastic strain of the axial screw dislocation, which produces a corresponding Eshelby Twist19,20 in the nanowires. In addition to opening up new opportunities for tailoring the properties of nanomaterials, these chiral branched nanowires also provide a direct visualization of the Eshelby Twist.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Odom, T. W., Huang, J. L., Kim, P. & Lieber, C. M. Atomic structure and electronic properties of single-walled carbon nanotubes. Nature 391, 62–64 (1998).
Wildoer, J. W. G., Venema, L. C., Rinzler, A. G., Smalley, R. E. & Dekker, C. Electronic structure of atomically resolved carbon nanotubes. Nature 391, 59–62 (1998).
Kondo, Y. & Takayanagi, K. Synthesis and characterization of helical multi-shell gold nanowires. Science 289, 606–608 (2000).
Che, S. et al. Synthesis and characterization of chiral mesoporous silica. Nature 429, 281–284 (2004).
Wu, Y. Y. et al. Composite mesostructures by nano-confinement. Nature Mater. 3, 816–822 (2004).
Gao, P. X. et al. Conversion of zinc oxide nanobelts into superlattice-structured nanohelices. Science 309, 1700–1704 (2005).
Meister, S. et al. Synthesis and characterization of phase-change nanowires. Nano Lett. 6, 1514–1517 (2006).
Zhang, H. F., Wang, C. M. & Wang, L. S. Helical crystalline SiC/SiO2 core–shell nanowires. Nano Lett. 2, 941–944 (2002).
Manna, L., Milliron, D. J., Meisel, A., Scher, E. C. & Alivisatos, A. P. Controlled growth of tetrapod-branched inorganic nanocrystals. Nature Mater. 2, 382–385 (2003).
Dick, K. A. et al. Synthesis of branched ‘nanotrees’ by controlled seeding of multiple branching events. Nature Mater. 3, 380–384 (2004).
Wang, D., Qian, F., Yang, C., Zhong, Z. H. & Lieber, C. M. Rational growth of branched and hyperbranched nanowire structures. Nano Lett. 4, 871–874 (2004).
May, S. J., Zheng, J. G., Wessels, B. W. & Lauhon, L. J. Dendritic nanowire growth mediated by a self-assembled catalyst. Adv. Mater. 17, 598–602 (2005).
Zhu, J. et al. Hyperbranched lead selenide nanowire networks. Nano Lett. 7, 1095–1099 (2007).
Fardy, M., Hochbaum, A. I., Goldberger, J., Zhang, M. M. & Yang, P. D. Synthesis and thermoelectrical characterization of lead chalcogenide nanowires. Adv. Mater. 19, 3047–3051 (2007).
Bierman, M. J., Lau, Y. K. A. & Jin, S. Hyperbranched PbS and PbSe nanowires and the effect of hydrogen gas on their synthesis. Nano Lett. 7, 2907–2912 (2007).
Ge, J. P. et al. Orthogonal PbS nanowire arrays and networks and their Raman scattering behaviour. Chem. Eur. J. 11, 1889–1894 (2005).
Sun, B. Q., Marx, E. & Greenham, N. C. Photovoltaic devices using blends of branched CdSe nanoparticles and conjugated polymers. Nano Lett. 3, 961–963 (2003).
Cui, Y., Banin, U., Bjork, M. T. & Alivisatos, A. P. Electrical transport through a single nanoscale semiconductor branch point. Nano Lett. 5, 1519–1523 (2004).
Eshelby, J. D. Screw dislocations in thin rods. J. Appl. Phys. 24, 176–179 (1953).
Bierman, M. J., Lau, Y. H. A., Kvit, A. V., Schmitt, A. L. & Jin, S. Dislocation-driven nanowire growth and Eshelby Twist. Science 320, 1060–1063 (2008).
Wagner, R. S. & Ellis, W. C. Vapour–liquid–solid mechanism of single crystal growth. Appl. Phys. Lett. 4, 89–90 (1964).
Lieber, C. M. Nanoscale science and technology: Building a big future from small things. MRS Bull. 28, 486–491 (2003).
Yang, P. The chemistry and physics of semiconductor nanowires. MRS Bull. 30, 85–91 (2005).
Sears, G. W. Twist in lithium fluoride whiskers. J. Chem. Phys. 31, 53–54 (1959).
Sears, G. W., DeVries, R. C. & Huffine, C. Twist in alumina whiskers. J. Chem. Phys. 34, 2142–2143 (1961).
Veblen, D. R. & Post, J. E. A TEM study of fibrous cuprite (chalcotrichite): microstructures and growth mechanisms. Am. Mineral. 68, 790–803 (1983).
Drum, C. M. Twist and axial imperfections in filamentary crystals of aluminum nitride. II. J. Appl. Phy. 36, 824–829 (1965).
Gerber, C., Anselmetti, D., Bednorz, J. G., Mannhart, J. & Schlom, D. G. Screw dislocations in high-Tc films. Nature 350, 279–280 (1991).
Acknowledgements
Y.C. acknowledges support from the Stanford Global Energy and Climate Project and the Center for Probing the Nanoscale (CPN) with National Science Foundation (NSF) grant PHY-0425897. J.Z. is a CPN Fellow. W.D.N. acknowledges support by the Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under grant DE-FG02-04ER46163.
Author information
Authors and Affiliations
Contributions
J.Z. and Y.C. conceived and designed the experiments. J.Z., A.F.M. and HP. performed the experiments. D.M.B. and W.D.N. performed simulation. J.Z., A.F.M., W.D.N. and Y.C. co-wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Rights and permissions
About this article
Cite this article
Zhu, J., Peng, H., Marshall, A. et al. Formation of chiral branched nanowires by the Eshelby Twist. Nature Nanotech 3, 477–481 (2008). https://doi.org/10.1038/nnano.2008.179
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2008.179
This article is cited by
-
Reconfiguring nucleation for CVD growth of twisted bilayer MoS2 with a wide range of twist angles
Nature Communications (2024)
-
Non-spherical gold nanoparticles enhanced fluorescence of carbon dots for norovirus-like particles detection
Journal of Biological Engineering (2023)
-
Enantioselectivity of discretized helical supramolecule consisting of achiral cobalt phthalocyanines via chiral-induced spin selectivity effect
Nature Communications (2023)
-
Twisting, untwisting, and retwisting of elastic Co-based nanohelices
Nature Communications (2023)
-
Breaking the symmetry of colloidal 2D nanoplatelets: Twist induced quantum coupling
Nano Research (2023)