Abstract
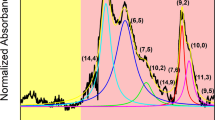
Solubilizing and purifying carbon nanotubes remains one of the foremost technological hurdles in their investigation and application. We report a dramatic improvement in the preparation of single-walled carbon nanotube solutions based on the ability of specific aromatic polymers to efficiently disperse certain nanotube species with a high degree of selectivity. Evidence of this is provided by optical absorbance and photoluminescence excitation spectra, which show suspensions corresponding to up to ∼60% relative concentration of a single species of isolated nanotubes with fluorescence quantum yields of up to 1.5%. Different polymers show the ability to discriminate between nanotube species in terms of either diameter or chiral angle. Modelling suggests that rigid-backbone polymers form ordered molecular structures surrounding the nanotubes with n-fold symmetry determined by the tube diameter.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dresselhaus, M. S., Dresselhaus, G. & Avouris, P. Carbon Nanotubes (Springer, Berlin, 2001).
Saito, R., Fujita, M., Dresselhaus, G. & Dresselhaus, M. S. Electronic structure of chiral graphene tubules. Appl. Phys. Lett. 60, 2204–2206 (1992).
Nish, A. & Nicholas, R. J. Temperature induced restoration of fluorescence from oxidised single-walled carbon nanotubes in aqueous sodium dodecylsulfate solution. Phys. Chem. Chem. Phys. 8, 3547–3551 (2006).
Matarredona, O. et al. Dispersion of single-walled carbon nanotubes in aqueous solutions of the anionic surfactant NaDDBS. J. Phys. Chem. B 107, 13357–13367 (2003).
O'Connell, M. J. et al. Band gap fluorescence from individual single-walled carbon nanotubes. Science 297, 593–596 (2002).
Arnold, M. S., Green, A. A., Hulvat, J. F., Strupp, S. I. & Hersam, M. C. Sorting carbon nanotubes by electronic structure using density differentiation. Nature Nanotech. 1, 60–65 (2006).
Bachilo, S. M. et al. Structure-assigned optical spectra of single-walled carbon nanotubes. Science 298, 2361–2366 (2002).
Zheng, M. et al. DNA-assisted dispersion and separation of carbon nanotubes. Nature Mater. 2, 338–342 (2003).
Zheng M. & Diner, B. A. Solution redox chemistry of carbon nanotubes. J. Am. Chem. Soc. 126, 15490–15494 (2004).
O'Connell, M. J. et al. Reversible water-solubilization of single-walled carbon nanotubes by polymer wrapping. Chem. Phys. Lett. 342, 265–271 (2001).
Rice, N. A., Soper, K., Zhou, N., Merschrod, E. & Zhao, Y. Dispersing as-prepared single-walled carbon nanotube powders with linear conjugated polymers. Chem. Commun. 47, 4937–4939 (2006).
Wei, C. Radius and chirality dependent conformation of polymer molecule at nanotube interface. Nano. Lett. 6, 1627–1631 (2006).
Chen, J. et al. Noncovalent engineering of carbon nanotube surfaces by rigid, functional conjugated polymers. J. Am. Chem. Soc. 124, 9034–9035 (2002).
Yang, M., Koutsos, V. & Zaiser, M. Interactions between polymers and carbon nanotubes: A molecular dynamics study. J. Phys. Chem. B 109, 10009–10014 (2005).
McCarthy, B. et al. A microscopic and spectroscopic study of interactions between carbon nanotubes and a conjugated polymer. J. Phys. Chem. B 106, 2210–2216 (2002).
Weisman, R. B. & Bachilo, S. M. Dependence of optical transition energies on structure for single-walled carbon nanotubes in aqueous suspension: An empirical Kataura plot. Nano. Lett. 3, 1235–1238 (2003).
Ando, T. Excitons in carbon nanotubes revisited: Dependence on diameter, Aharonov-Bohm flux, and strain. J. Phys. Soc. Jpn. 73, 3351–3363 (2004).
Jones, M. et al. Analysis of photoluminescence from solubilized single-walled carbon nanotubes. Phys. Rev. B 71, 115426 (2005).
Miyauchi, Y., Oba, M. & Maruyama, S. Cross-polarized optical absorption of single-walled nanotubes by polarized photoluminescence excitation spectroscopy. Phys. Rev. B 74, 205440 (2006).
Ponder, J. W. & Richards, F. M. An efficient Newton-like method for molecular mechanics energy minimization of large molecules. J. Comput. Chem. 8, 1016–1024 (1987).
Allinger, N. L., Yuh, Y. H. & Lii, J. H. Molecular mechanics. The MM3 force field for hydrocarbons. 1. J. Am. Chem. Soc. 111, 8551–8566 (1989).
Hornbostal, B., Haluska, M., Cech, J., Dettlaff, U. & Roth, S. Arc discharge and laser ablation synthesis of single-walled carbon nanotubes. NATO Science Series II: Mathematics, Physics and Chemistry 222, 1–18 (2005).
Reich, S., Thomson, C. & Robertson, J. Exciton resonances quench the photoluminescence of zigzag carbon nanotubes. Phys. Rev. Lett. 95, 077402 (2005).
Oyama, Y. et al. Photoluminescence intensity of single-wall carbon nanotubes. Carbon 44, 873–879 (2006).
Huang, X. Y., McLean, R. S. & Zheng, M. High-resolution length sorting and purification of DNA-wrapped carbon nanotubes by size-exclusion chromatography. Anal. Chem. 77, 6225–6228 (2005).
Lefebvre, J., Austing, D. G., Bond, J. & Finnie, P. Photoluminescence imaging of suspended single-walled carbon nanotubes. Nano Lett. 6, 1603–1608 (2006).
Weisman, R. B., Bachilo, S. M. & Tsyboulski, D. Fluorescence spectroscopy of single-walled carbon nanotubes in aqueous suspension. Appl. Phys. A 78, 1111–1116 (2004).
Nikolaev, P. et al. Gas-phase catalytic growth of single-walled carbon nanotubes from carbon monoxide. Chem. Phys. Lett. 313, 91–97 (1999).
Bachilo, S. M. et al. Narrow (n,m)-distribution of single-walled carbon nanotubes grown using a solid supported catalyst. J. Am. Chem. Soc. 125, 11186–11187 (2003).
Acknowledgements
The authors acknowledge the Basic Technology Program of the Engineering and Physical Sciences Research Council for their financial support and C. Pears, in Biochemistry, at the University of Oxford for use of ultracentrifuge facilities. Jeong-Yuan Hwang would like to acknowledge the National Science Council of Taiwan and the Thousand Mile Horse Program for their financial support.
Author information
Authors and Affiliations
Contributions
A.N. performed the data analysis and co-wrote the paper. J.-Y.H. prepared the samples and did the optical absorbance and PL measurements. J.D. carried out the computer simulations and took the Raman spectra. R.J.N. coordinated the project and co-wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
We have applied for a British patent on 18 May 2007 entitled 'Method of carbon nanotube selection'. Some of the results and methods used in this paper were added to our patent filing.
Supplementary information
Supplementary Information
Supplementary figures S1-S3 (PDF 717 kb)
Rights and permissions
About this article
Cite this article
Nish, A., Hwang, JY., Doig, J. et al. Highly selective dispersion of single-walled carbon nanotubes using aromatic polymers. Nature Nanotech 2, 640–646 (2007). https://doi.org/10.1038/nnano.2007.290
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2007.290