Abstract
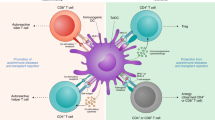
We have shown that nanoparticles (NPs) can be used as ligand-multimerization platforms to activate specific cellular receptors in vivo. Nanoparticles coated with autoimmune disease-relevant peptide-major histocompatibility complexes (pMHC) blunted autoimmune responses by triggering the differentiation and expansion of antigen-specific regulatory T cells in vivo. Here, we define the engineering principles impacting biological activity, detail a synthesis process yielding safe and stable compounds, and visualize how these nanomedicines interact with cognate T cells. We find that the triggering properties of pMHC–NPs are a function of pMHC intermolecular distance and involve the sustained assembly of large antigen receptor microclusters on murine and human cognate T cells. These compounds show no off-target toxicity in zebrafish embryos, do not cause haematological, biochemical or histological abnormalities, and are rapidly captured by phagocytes or processed by the hepatobiliary system. This work lays the groundwork for the design of ligand-based NP formulations to re-program in vivo cellular responses using nanotechnology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Tsai, S. et al. Reversal of autoimmunity by boosting memory-like autoregulatory T cells. Immunity 32, 568–580 (2010).
Clemente-Casares, X. et al. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature 530, 434–440 (2016).
Clemente-Casares, X., Tsai, S., Yang, Y. & Santamaria, P. Peptide-MHC-based nanovaccines for the treatment of autoimmunity: a “one size fits all” approach? J. Mol. Med. 89, 733–742 (2011).
McLarnon, A. IBD: regulatory T-cell therapy is a safe and well-tolerated potential approach for treating refractory Crohn's disease. Nat. Rev. Gastroenterol. Hepatol. 9, 509–516 (2012).
Desreumaux, P. et al. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn's disease. Gastroenterology 143, 1207–1217 (2012).
Xie, J. et al. One-pot synthesis of monodisperse iron oxide nanoparticles for potential biomedical applications. Pure Appl. Chem. 78, 1003–1014 (2006).
Rojo, J. M. & Portoles, P. A symmetrical view of the T-cell receptor-CD3 complex. Immunol. Today 12, 377–378 (1991).
Fernandez-Miguel, G. et al. Multivalent structure of an αβT cell receptor. Proc. Natl Acad. Sci. USA 96, 1547–1552 (1996).
Arechaga, I. et al. Structural characterization of the TCR complex by electron microscopy. Int. Immunol. 22, 897–903 (2010).
Scholten, K. B. et al. Preservation and redirection of HPV16E7-specific T cell receptors for immunotherapy of cervical cancer. Clin. Immunol. 114, 119–129 (2005).
Schamel, W. W. & Alarcon, B. Organization of the resting TCR in nanoscale oligomers. Immunol. Rev. 251, 13–20 (2013).
Lillemeier, B. F. et al. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat. Immunol. 11, 90–96 (2010).
Zhong, L. et al. NSOM/QD-based direct visualization of CD3-induced and CD28-enhanced nanospatial coclustering of TCR and coreceptor in nanodomains in T cell activation. PLoS ONE 4, e5945 (2009).
Yokosuka, T. et al. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat. Immunol. 6, 1253–1262 (2005).
Choudhuri, K. & Dustin, M. L. Signaling microdomains in T cells. FEBS Lett. 584, 4823–4831 (2010).
Sherman, E. et al. Functional nanoscale organization of signaling molecules downstream of the T cell antigen receptor. Immunity 35, 705–720 (2011).
Weisser, S. B., van Rooijen, N. & Sly, L. M. Depletion and reconstitution of macrophages in mice. J. Vis. Exp. 66, e4105 (2012).
Gagliani, N. et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat. Med. 19, 739–746 (2013).
Roncarolo, M. G., Gregori, S., Bacchetta, R. & Battaglia, M. Tr1 cells and the counter-regulation of immunity: natural mechanisms and therapeutic applications. Curr. Top Microbiol. Immunol. 380, 39–68 (2014).
Gu, L., Fang, R. H., Sailor, M. J. & Park, J. H. In vivo clearance and toxicity of monodisperse iron oxide nanocrystals. ACS Nano 6, 4947–4954 (2012).
Kolosnjaj-Tabi, J. et al. The one year fate of iron oxide coated gold nanoparticles in mice. ACS Nano 9, 7925–7939 (2015).
Chou, L. Y., Zagorovsky, K. & Chan, W. C. DNA assembly of nanoparticle superstructures for controlled biological delivery and elimination. Nat. Nanotech. 9, 148–155 (2014).
Alexis, F., Pridgen, E., Molnar, L. K. & Farokhzad, O. C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 5, 505–515 (2008).
Borchard, G. & Kreuter, J. The role of serum complement on the organ distribution of intravenously administered poly (methyl methacrylate) nanoparticles: effects of pre-coating with plasma and with serum complement. Pharm. Res. 13, 1055–1058 (1996).
Armstrong, T. I., Davies, M. C. & Illum, L. Human serum albumin as a probe for protein adsorption to nanoparticles: relevance to biodistribution. J. Drug Target 4, 389–398 (1997).
Roohi, F., Lohrke, J., Ide, A., Schutz, G. & Dassler, K. Studying the effect of particle size and coating type on the blood kinetics of superparamagnetic iron oxide nanoparticles. Int. J. Nanomed. 7, 4447–4458 (2012).
Bourrinet, P. et al. Preclinical safety and pharmacokinetic profile of ferumoxtran-10, an ultrasmall superparamagnetic iron oxide magnetic resonance contrast agent. Invest. Radiol. 41, 313–324 (2006).
Varallyay, P. et al. Comparison of two superparamagnetic viral-sized iron oxide particles ferumoxides and ferumoxtran-10 with a gadolinium chelate in imaging intracranial tumors. AJNR Am. J. Neuroradiol. 23, 510–519 (2002).
Scholer, N. et al. Effect of solid lipid nanoparticles (SLN) on cytokine production and the viability of murine peritoneal macrophages. J. Microencapsul. 17, 639–650 (2000).
Scholer, N., Hahn, H., Muller, R. H. & Liesenfeld, O. Effect of lipid matrix and size of solid lipid nanoparticles (SLN) on the viability and cytokine production of macrophages. Int. J. Pharm. 231, 167–176 (2002).
Fifis, T. et al. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J. Immunol. 173, 3148–3154 (2004).
Shvedova, A. A. et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, L698–L708 (2005).
Vallhov, H. et al. The importance of an endotoxin-free environment during the production of nanoparticles used in medical applications. Nano Lett. 6, 1682–1686 (2006).
Mottram, P. L. et al. Type 1 and 2 immunity following vaccination is influenced by nanoparticle size: formulation of a model vaccine for respiratory syncytial virus. Mol. Pharm. 4, 73–84 (2007).
Huppa, J. B. et al. TCR-peptide-MHC interactions in situ show accelerated kinetics and increased affinity. Nature 463, 963–967 (2010).
Bunnell, S. C. et al. T cell receptor ligation induces the formation of dynamically regulated signaling assemblies. J. Cell Biol. 158, 1263–1275 (2002).
Gil, D., Schamel, W. W., Montoya, M., Sanchez-Madrid, F. & Alarcon, B. Recruitment of Nck by CD3ɛ reveals a ligand-induced conformational change essential for T cell receptor signaling and synapse formation. Cell 109, 901–912 (2002).
Minguet, S., Swamy, M., Alarcon, B., Luescher, I. F. & Schamel, W. W. Full activation of the T cell receptor requires both clustering and conformational changes at CD3. Immunity 26, 43–54 (2007).
Martinez-Martin, N. et al. Cooperativity between T cell receptor complexes revealed by conformational mutants of CD3ɛ. Sci. Signal. 2, ra43 (2009).
McKeithan, T. W. Kinetic proofreading in T-cell receptor signal transduction. Proc. Natl Acad. Sci. USA 92, 5042–5046 (1995).
Valitutti, S., Muller, S., Cella, M., Padovan, E. & Lanzavecchia, A. Serial triggering of many T-cell receptors by a few peptide–MHC complexes. Nature 375, 148–151 (1995).
Katz, J. D., Wang, B., Haskins, K., Benoist, C. & Mathis, D. Following a diabetogenic T cell from genesis through pathogenesis. Cell 74, 1089–1100 (1993).
Verdaguer, J. et al. Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J. Exp. Med. 186, 1663–1676 (1997).
Han, B. et al. Developmental control of CD8+ T cell-avidity maturation in autoimmune diabetes. J. Clin. Invest. 115, 1879–1887 (2005).
Garboczi, D. N., Hung, D. T. & Wiley, D. C. HLA-A2-peptide complexes: refolding and crystallization of molecules expressed in Escherichia coli and complexed with single antigenic peptides. Proc. Natl Acad. Sci. USA 89, 3429–3433 (1992).
Altman, J. D. et al. Phenotypic analysis of antigen-specific T lymphocytes. Science 274, 94–96 (1996).
Yu, Y. Y., Netuschil, N., Lybarger, L., Connolly, J. M. & Hansen, T. H. Cutting edge: single-chain trimers of MHC class I molecules form stable structures that potently stimulate antigen-specific T cells and B cells. J. Immunol. 168, 3145–3149 (2002).
Holst, J. et al. Generation of T-cell receptor retrogenic mice. Nat. Protoc. 1, 406–417 (2006).
Amrani, A. et al. Progression of autoimmune diabetes driven by avidity maturation of a T-cell population. Nature 406, 739–742 (2000).
Stratmann, T. et al. The I-Ag7 MHC class II molecule linked to murine diabetes is a promiscuous peptide binder. J. Immunol. 165, 3214–3225 (2000).
Perrault, S. D., Walkey, C., Jennings, T., Fischer, H. C. & Chan, W. C. Mediating tumor targeting efficiency of nanoparticles through design. Nano Lett. 9, 1909–1915 (2009).
Xie, J., Xu, C., Kohler, N., Hou, Y. & Sun, S. Controlled PEGylation of monodisperse Fe3O4 nanoparticles for reduced non-specific uptake by macrophage cells. Adv. Mater. 19, 3163–3166 (2007).
Xu, C. & Sun, S. Monodisperse magnetic nanoparticles for biomedical applications. Polym. Int. 56, 821–826 (2007).
Afonin, K. A. et al. Design and self-assembly of siRNA-functionalized RNA nanoparticles for use in automated nanomedicine. Nat. Protoc. 6, 2022–2034 (2011).
Li, Y. & Boraschi, D. Endotoxin contamination: a key element in the interpretation of nanosafety studies. Nanomedicine 11, 269–287 (2016).
Nikolic, T., Geutskens, S. B., van Rooijen, N., Drexhage, H. A. & Leenen, P. J. Dendritic cells and macrophages are essential for the retention of lymphocytes in (peri)-insulitis of the nonobese diabetic mouse: a phagocyte depletion study. Lab. Invest. 85, 487–501 (2005).
Calderon, B., Suri, A. & Unanue, E. R. CD4+ T-cell-induced diabetes, macrophages are the final effector cells that mediate islet β-cell killing: studies from an acute model. Am. J. Pathol. 169, 2137–2147 (2006).
Mandrell, D. et al. Automated zebrafish chorion removal and single embryo placement: optimizing throughput of zebrafish developmental toxicity screens. J. Lab. Autom. 17, 66–74 (2012).
Truong, L., Harper, S. L. & Tanguay, R. L. Evaluation of embryotoxicity using the zebrafish model. Methods Mol. Biol. 691, 271–279 (2011).
Truong, L. et al. Multidimensional in vivo hazard assessment using zebrafish. Toxicol. Sci. 137, 212–233 (2014).
Acknowledgements
We thank S. Thiessen, J. Erickson and J. Luces for technical assistance, and L. Kennedy for flow cytometry. We acknowledge T. DiLorenzo for providing JurMA cells, H. Benediktsson for structural analyses of kidney TEM, R. Interior for amino acid analysis, W. White and D. Cramb for assistance with GD-mass spectrometry, FTIR and DLS, T. Furstenhaupt and W. Dong for SEBD and TEM, J.M. Rebled, A. Garcia, R. Rivera and A. Martínez from the TEM–SEM unit from the University of Barcelona (CCiT-UB) for TEM analyses of human T-cell clone:pMHC–NP conjugates, and the CMHD Unit at the Lunenfeld–Tanenbaum Institute for haematology and biochemistry. This work was funded by the Collaborative Health Research Program of the Canadian Institutes of Health Research (CIHR) and the Natural Sciences and Engineering Research Council of Canada, the Instituto de Investigaciones Sanitarias Carlos III, the Ministerio de Economia y Competitividad of Spain (MINECO), and the Sardà Farriol Research Programme. X.C.C. was supported by studentships from the AXA Research Fund and the endMS network. K.S. is funded by Eyes’ High/Alberta Innovates-Technology Futures, Alberta Innovates–Health Solutions (AI-HS) and Banting-CIHR fellowships. C.S.U. is supported by AI-HS and Banting-CIHR fellowhips. R.H.N. is supported by studentships from AI-HS and CIHR. S.W.L. was partially supported by a studentship from Fonds de Recherche du Quebec - Nature et Technologies. J.B. was supported by a Rio Hortega fellowship from the Ministry of Economy and Competitiveness of Spain and by a fellowship from the European Association for the Study of Diabetes (EASD). P.Serra is a Ramon y Cajal investigator supported by a Juvenile Diabetes Research Foundation Career Development Award. P.Santamaria is Scientist of the Alberta Innovates-Health Solutions and a scholar of the IISCIII. The JMDRC is supported by the Canadian Diabetes Association (CDA).
Author information
Authors and Affiliations
Contributions
S.S. and Y.Y. developed and produced all the pMHC–NPs, and data for Figs 1, 2a–j, 4a–c, 4e–g and Supplementary Figs 1, 4d–f,g, 5a and 7b, in collaboration with K.S.; K.S. produced data for Figs 2l, 4d, and Supplementary Figs 4a–c and 8. X.C. produced data for Figs 3e–h and 4i and Supplementary Figs 3 and 7a, in collaboration with J.Y.; P. Solé produced the JurMA TCR/mCDA transfectants and produced the data for Fig. 2k. J.Y. produced the data for Supplementary Fig. 4c. C.S.U. and R.H.N. produced murine pMHCs for this study. C.F. purified human pMHCs. J.B., A.C. and P. Serra produced data for Fig. 5. Q.D., F.S. and W.C.W.C. produced data for Fig. 6 and Supplementary Fig. 5. S.W.L. and A.K. generated all the mathematical models and produced Fig. 3a–d and Supplementary Figs 9 and 10. P.D. and M.A. contributed expertise in confocal microscopy and SEM. R.T. generated the zebrafish embryo toxicology data for Supplementary Fig. 6. S.N. carried out the multi-organ histopathology. P. Santamaria designed and supervised the study and wrote the manuscript with the assistance of S.S.
Corresponding authors
Ethics declarations
Competing interests
P. Santamaria is scientific founder of Parvus Therapeutics Inc. and has a financial interest in the company.
Supplementary information
Supplementary information
Supplementary information (PDF 4280 kb)
Rights and permissions
About this article
Cite this article
Singha, S., Shao, K., Yang, Y. et al. Peptide–MHC-based nanomedicines for autoimmunity function as T-cell receptor microclustering devices. Nature Nanotech 12, 701–710 (2017). https://doi.org/10.1038/nnano.2017.56
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2017.56
This article is cited by
-
The therapeutic potential of immunoengineering for systemic autoimmunity
Nature Reviews Rheumatology (2024)
-
A T follicular helper cell origin for T regulatory type 1 cells
Cellular & Molecular Immunology (2023)
-
Engineering nanomaterial physical characteristics for cancer immunotherapy
Nature Reviews Bioengineering (2023)
-
Targeted modulation of immune cells and tissues using engineered biomaterials
Nature Reviews Bioengineering (2023)
-
Therapeutic induction of antigen-specific immune tolerance
Nature Reviews Immunology (2023)