Abstract
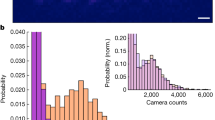
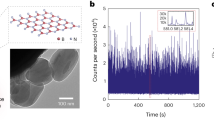
Fluid phase transitions inside single, isolated carbon nanotubes are predicted to deviate substantially from classical thermodynamics. This behaviour enables the study of ice nanotubes and the exploration of their potential applications. Here we report measurements of the phase boundaries of water confined within six isolated carbon nanotubes of different diameters (1.05, 1.06, 1.15, 1.24, 1.44 and 1.52 nm) using Raman spectroscopy. The results reveal an exquisite sensitivity to diameter and substantially larger temperature elevations of the freezing transition (by as much as 100 °C) than have been theoretically predicted. Dynamic water filling and reversible freezing transitions were marked by 2–5 cm−1 shifts in the radial breathing mode frequency, revealing reversible melting bracketed to 105–151 °C and 87–117 °C for 1.05 and 1.06 nm single-walled carbon nanotubes, respectively. Near-ambient phase changes were observed for 1.44 and 1.52 nm nanotubes, bracketed between 15–49 °C and 3–30 °C, respectively, whereas the depression of the freezing point was observed for the 1.15 nm nanotube between −35 and 10 °C. We also find that the interior aqueous phase reversibly decreases the axial thermal conductivity of the nanotube by as much as 500%, allowing digital control of the heat flux.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Shultz, M. J., Vu, T. H., Meyer, B. & Bisson, P. Water: a responsive small molecule. Acc. Chem. Res. 45, 15–22 (2012).
Iijima, S. & Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 363, 603–605 (1993).
Koga, K., Gao, G. T., Tanaka, H. & Zeng, X. C. Formation of ordered ice nanotubes inside carbon nanotubes. Nature 412, 802–805 (2001).
Hummer, G., Rasaiah, J. C. & Noworyta, J. P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 414, 188–190 (2001).
Zhao, Y. et al. Individual water-filled single-walled carbon nanotubes as hydroelectric power converters. Adv. Mater. 20, 1772–1776 (2008).
Fornasiero, F. et al. Ion exclusion by sub-2-nm carbon nanotube pores. Proc. Natl Acad. Sci. USA 105, 17250–17255 (2008).
Walther, J. H., Ritos, K., Cruz-Chu, E. R., Megaridis, C. M. & Koumoutsakos, P. Barriers to superfast water transport in carbon nanotube membranes. Nano Lett. 13, 1910–1914 (2013).
Noy, A. et al. Nanofluidics in carbon nanotubes. Nano Today 2, 22–29 (2007).
Perez, M. Gibbs–Thomson effects in phase transformations. Scr. Mater. 52, 709–712 (2005).
Christenson, H. K. Confinement effects on freezing and melting. J. Phys. Condens. Matter. 13, R95–R133 (2001).
Maniwa, Y. et al. Phase transition in confined water inside carbon nanotubes. J. Phys. Soc. Jpn 71, 2863–2866 (2002).
Takaiwa, D., Hatano, I., Koga, K. & Tanaka, H. Phase diagram of water in carbon nanotubes. Proc. Natl Acad. Sci. USA 105, 39–43 (2008).
Bai, J., Wang, J. & Zeng, X. C. Multiwalled ice helixes and ice nanotubes. Proc. Natl Acad. Sci. USA 103, 19664–19667 (2006).
Koga, K., Parra, R. D., Tanaka, H. & Zeng, X. C. Ice nanotube: what does the unit cell look like? J. Chem. Phys. 113, 5037–5040 (2000).
Kyakuno, H. et al. Confined water inside single-walled carbon nanotubes: global phase diagram and effect of finite length. J. Chem. Phys. 134, 244501 (2011).
Mann, D. J. & Halls, M. D. Water alignment and proton conduction inside carbon nanotubes. Phys. Rev. Lett. 90, 195503 (2003).
Maniwa, Y. et al. Ordered water inside carbon nanotubes: formation of pentagonal to octagonal ice-nanotubes. Chem. Phys. Lett. 401, 534–538 (2005).
Mikami, F., Matsuda, K., Kataura, H. & Maniwa, Y. Dielectric properties of water inside single-walled carbon nanotubes. ACS Nano 3, 1279–1287 (2009).
Maniwa, Y. et al. Water-filled single-wall carbon nanotubes as molecular nanovalves. Nat. Mater. 6, 135–141 (2007).
Shiomi, J., Kimura, T. & Maruyama, S. Molecular dynamics of ice-nanotube formation inside carbon nanotubes. J. Phys. Chem. C 111, 12188–12193 (2007).
Ghosh, S., Ramanathan, K. V. & Sood, A. K. Water at nanoscale confined in single-walled carbon nanotubes studied by NMR. Europhys. Lett. 65, 678–684 (2004).
Luo, C., Fa, W., Zhou, J., Dong, J. & Zeng, X. C. Ferroelectric ordering in ice nanotubes confined in carbon nanotubes. Nano Lett. 8, 2607–2612 (2008).
Pascal, T. A., Goddard, W. A. & Jung, Y. Entropy and the driving force for the filling of carbon nanotubes with water. Proc. Natl Acad. Sci. USA 108, 11794–11798 (2011).
Striolo, A., Chialvo, A. A., Gubbins, K. E. & Cummings, P. T. Water in carbon nanotubes: adsorption isotherms and thermodynamic properties from molecular simulation. J. Chem. Phys. 122, 234712 (2005).
Ghosh, S., Sood, A. K. & Kumar, N. Carbon nanotube flow sensors. Science 299, 1042–1044 (2003).
Lee, C. Y., Choi, W., Han, J.-H. & Strano, M. S. Coherence resonance in a single-walled carbon nanotube ion channel. Science 329, 1320–1324 (2010).
Holt, J. K. et al. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 312, 1034–1037 (2006).
Corry, B. Designing carbon nanotube membranes for efficient water desalination. J. Phys. Chem. B 112, 1427–1434 (2008).
Striolo, A. The mechanism of water diffusion in narrow carbon nanotubes. Nano Lett. 6, 633–639 (2006).
Cabeza, L. F., Mehling, H., Hiebler, S. & Ziegler, F. Heat transfer enhancement in water when used as PCM in thermal energy storage. Appl. Therm. Eng. 22, 1141–1151 (2002).
Thomas, M. & Corry, B. Thermostat choice significantly influences water flow rates in molecular dynamics studies of carbon nanotubes. Microfluid. Nanofluid. 18, 41–47 (2015).
Werder, T., Walther, J. H., Jaffe, R. L., Halicioglu, T. & Koumoutsakos, P. On the water–carbon interaction for use in molecular dynamics simulations of graphite and carbon nanotubes. J. Phys. Chem. B 107, 1345–1352 (2003).
Choi, W., Lee, C. Y., Ham, M.-H., Shimizu, S. & Strano, M. S. Dynamics of simultaneous, single ion transport through two single-walled carbon nanotubes: observation of a three-state system. J. Am. Chem. Soc. 133, 203–205 (2011).
Choi, W. et al. Diameter-dependent ion transport through the interior of isolated single-walled carbon nanotubes. Nat. Commun. 4, 2397 (2013).
Jorio, A. et al. Structural (n,m) determination of isolated single-wall carbon nanotubes by resonant Raman scattering. Phys. Rev. Lett. 86, 1118–1121 (2001).
Jorio, A. et al. G-band resonant Raman study of 62 isolated single-wall carbon nanotubes. Phys. Rev. B 65, 23–27 (2002).
Li, F. et al. Identification of the constituents of double-walled carbon nanotubes using Raman spectra taken with different laser-excitation energies. J. Mater. Res. 18, 1251–1258 (2003).
Cambré, S., Schoeters, B., Luyckx, S., Goovaerts, E. & Wenseleers, W. Experimental observation of single-file water filling of thin single-wall carbon nanotubes down to chiral index (5,3). Phys. Rev. Lett. 104, 207401 (2010).
Wenseleers, W., Cambré, S., Čulin, J., Bouwen, A. & Goovaerts, E. Effect of water filling on the electronic and vibrational resonances of carbon nanotubes: characterizing tube opening by Raman spectroscopy. Adv. Mater. 19, 2274–2278 (2007).
Longhurst, M. J. & Quirke, N. The environmental effect on the radial breathing mode of carbon nanotubes. II. Shell model approximation for internally and externally adsorbed fluids. J. Chem. Phys. 125, 184705 (2006).
Zhang, Y., Son, H., Zhang, J., Kong, J. & Liu, Z. Laser-heating effect on Raman spectra of individual suspended single-walled carbon nanotubes. J. Phys. Chem. C 111, 1988–1992 (2007).
Zhang, Y., Xie, L., Zhang, J., Wu, Z. & Liu, Z. Temperature coefficients of Raman frequency of individual single-walled carbon nanotubes. J. Phys. Chem. C 111, 14031–14034 (2007).
Huang, F. et al. Temperature dependence of the Raman spectra of carbon nanotubes. J. Appl. Phys. 76, 2053–2055 (2000).
Wang, C. Y., Ru, C. Q. & Mioduchowski, A. Free vibration of multiwall carbon nanotubes. J. Appl. Phys. 97, 114323 (2005).
Efron, B. & Tibshirani, R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat. Sci. 1, 54–75 (1986).
Chiashi, S. et al. Water encapsulation control in individual single-walled carbon nanotubes by laser irradiation. J. Phys. Chem. Lett. 5, 408–412 (2014).
Sliwinska-Bartkowiak, M., Jazdzewska, M., Huang, L. L. & Gubbins, K. E. Melting behavior of water in cylindrical pores: carbon nanotubes and silica glasses. Phys. Chem. Chem. Phys. 10, 4909–4919 (2008).
Radhakrishnan, R., Gubbins, K. E. & Sliwinska-Bartkowiak, M. Global phase diagrams for freezing in porous media. J. Chem. Phys. 116, 1147–1155 (2002).
Chang, C. W., Okawa, D., Majumdar, A. & Zettl, A. Solid-state thermal rectifier. Science 314, 1121–1124 (2006).
Takahashi, K., Inoue, M. & Ito, Y . Defective carbon nanotube for use as a thermal rectifier. Jpn J. Appl. Phys. 49, 02BD12 (2010).
Acknowledgements
This work was supported in part by the U. S. Army Research Laboratory and the U. S. Army Research Office through the Institute for Soldier Nanotechnologies, under contract number W911NF-13-D-0001. We acknowledge support from the Shell-MIT-EI Energy Research Fund as well. We also acknowledge support from the National Science Foundation under Grant Number 1306529.
Author information
Authors and Affiliations
Contributions
K.V.A, S.S. and M.S.S. conceived and designed the experiments. K.V.A., S.S., L.W.D. and D.K. performed the experiments, analyzed the data, and contributed materials/analysis tools. K.V.A. and M.S.S. wrote the paper. All authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 3378 kb)
Rights and permissions
About this article
Cite this article
Agrawal, K., Shimizu, S., Drahushuk, L. et al. Observation of extreme phase transition temperatures of water confined inside isolated carbon nanotubes. Nature Nanotech 12, 267–273 (2017). https://doi.org/10.1038/nnano.2016.254
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2016.254
This article is cited by
-
Liquid-activated quantum emission from pristine hexagonal boron nitride for nanofluidic sensing
Nature Materials (2023)
-
Two-dimensional non-linear hydrodynamics and nanofluidics
Communications Physics (2023)
-
Differences in water and vapor transport through angstrom-scale pores in atomically thin membranes
Nature Communications (2022)
-
Simulations of Tapered Channel in Multilayer Graphene as Reverse Osmosis Membrane for Desalination
Journal of Wuhan University of Technology-Mater. Sci. Ed. (2022)
-
Freezing of few nanometers water droplets
Nature Communications (2021)