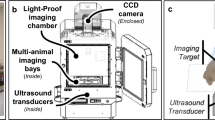
Abstract
Failure of cancer surgery to intraoperatively detect and eliminate microscopic residual disease (MRD) causes lethal recurrence and metastases, and the removal of important normal tissues causes excessive morbidity. Here, we show that a plasmonic nanobubble (PNB), a non-stationary laser pulse-activated nanoevent, intraoperatively detects and eliminates MRD in the surgical bed. PNBs were generated in vivo in head and neck cancer cells by systemically targeting tumours with gold colloids and locally applying near-infrared, low-energy short laser pulses, and were simultaneously detected with an acoustic probe. In mouse models, between 3 and 30 residual cancer cells and MRD (undetectable with current methods) were non-invasively detected up to 4 mm deep in the surgical bed within 1 ms. In resectable MRD, PNB-guided surgery prevented local recurrence and delivered 100% tumour-free survival. In unresectable MRD, PNB nanosurgery improved survival twofold compared with standard surgery. Our results show that PNB-guided surgery and nanosurgery can rapidly and precisely detect and remove MRD in simple intraoperative procedures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Meier, J. D., Oliver, D. A. & Varvares, M. A. Surgical margin determination in head and neck oncology: current clinical practice. The results of an International American Head and Neck Society Member Survey. Head Neck 27, 952–958 (2005).
de Carvalho, A. C. et al. Clinical significance of molecular alterations in histologically negative surgical margins of head and neck cancer patients. Oral. Oncol. 48, 240–248 (2012).
Loree, T. R. & Strong, E. W. Significance of positive margins in oral cavity squamous carcinoma. Am. J. Surg. 160, 410–414 (1990).
Looser, K. G., Shah, J. P. & Strong, E. W. The significance of ‘positive’ margins in surgically resected epidermoid carcinomas. Head Neck Surg. 1, 107–111 (1978).
Vikram, B., Strong, E. W., Shah, J. P. & Spiro, R. Failure at the primary site following multimodality treatment in advanced head and neck cancer. Head Neck Surg. 6, 720–723 (1984).
Leemans, C. R., Braakhuis, B. J. & Brakenhoff, R. H. The molecular biology of head and neck cancer. Nature Rev. Cancer 11, 9–22 (2011).
Haddad, R. I. & Shin, D. M. Recent advances in head and neck cancer. N. Engl. J. Med. 359, 1143–1154 (2008).
Calabrese, L. et al. Future challenges in head and neck cancer: from the bench to the bedside? Crit. Rev. Oncol. Hematol. 84, e90–e96 (2012).
Langendijk, J. A. et al. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J. Clin. Oncol. 26, 3770–3887 (2008).
Radosevich, J. A. (Ed.) Head and Neck Cancer: Current Perspectives, Advances and Challenges (Springer, 2013).
Vermorken, J. B. et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N. Engl. J. Med. 357, 1695–1704 (2007).
Wang, L. V. Multiscale photoacoustic microscopy and computed tomography. Nature Photon. 3, 503–509 (2009).
Jathoul, A. P. et al. Deep in vivo photoacoustic imaging of mammalian tissues using a tyrosinase-based genetic reporter. Nature Photon. 9, 239–246 (2015).
Kaiplavil, S. & Mandelis, A. Truncated-correlation photothermal coherence tomography for deep subsurface analysis. Nature Photon. 8, 635–642 (2014).
Lo Celso, C. et al. Live-animal tracking of individual haemotapoietic stem/progenitor cells in their niche. Nature 457, 92–96 (2009).
Upile, T. et al. Head and neck optical diagnostics: vision of the future of surgery. Head Neck Oncol. 1, 25 (2009).
Thorek, D. L., Ogirala, A., Beattie, B. J. & Grimm, J. Quantitative imaging of disease signatures through radioactive decay signal conversion. Nature Med. 19, 1345–1350 (2013).
Nguyen, Q. T. & Tsien, R. Y. Fluorescence-guided surgery with live molecular navigation—a new cutting edge. Nature Rev. 13, 653–662 (2013).
van Dam, G. M. et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate reseptor-α targeting: first in-human results. Nature Med. 17, 1315–1319 (2011).
Vahmeijer, A. L., Hutteman, M., van de Vorst, J. R., van de Velde, C. J. H. & Frangioni, J. V. Image-guided cancer surgery using near-infrared fluorescence. Nature Rev. Clin. Oncol. 10, 507–518 (2013).
Holt, D. et al. Intraoperative near-infrared imaging can distinguish cancer from normal tissue but not inflammation. PLoS ONE 9, e103342 (2014).
Troyan, S. L. et al. The FLARE intraoperative near-infrared fluorescence imaging system: a first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann. Surg. Oncol. 16, 2943–2952 (2009).
Qiu, L. et al. Multispectral scanning during endoscopy guides biopsy of dysplasia in Barrett's esophagus. Nature Med. 16, 603–606 (2010).
Ntziachristos, V. Clinical translation of optical and optoacoustic images. Phil. Trans. R. Soc. A. 369, 4666–4678 (2011).
De Boer, E. et al. Optical innovations in surgery. Br. J. Surg. 105, e56–e72 (2015).
Taruttis, A. & Ntziachristos, V. Advances in real-time multispectral optoacoustic imaging and its applications. Nature Photon. 9, 219–227 (2015).
Lukianova-Hleb, E. Y. et al. On-demand intracellular amplification of chemoradiation with cancer-specific plasmonic nanobubbles. Nature Med. 20, 778–784 (2014).
Hleb, E. Y. et al. LANTCET: elimination of solid tumor cells with photothermal bubbles generated around clusters of gold nanoparticles. Nanomedicine 3, 647–667 (2008).
Lukianova-Hleb, E. et al. Plasmonic nanobubbles as transient vapor nanobubbles generated around plasmonic nanoparticles. ACS Nano 4, 2109–2123 (2010).
Lukianova-Hleb, E. Y., Volkov, A. N., Wu, X. & Lapotko, D. O. Transient enhancement and spectral narrowing of the photothermal effect of plasmonic nanoparticles under pulsed excitation. Adv. Mater. 25, 772–776 (2013).
Kitz, M. et al. Vapor bubble generation around gold nano-particles and its application to damaging of cells. Biomed. Opt. Express 2, 291–304 (2011).
Pitsillides, C. M., Joe, E. K., Wei, X., Anderson, R. R. & Lin, C. P. Selective cell targeting with light-absorbing microparticles and nanoparticles. Biophys. J. 84, 4023–4032 (2003).
Lapotko, D. et al. Photothermal microscopy and laser ablation of leukemia cells targeted with gold nanoparticles. Proc. SPIE 5697, 82–89 (2005).
Lukianova-Hleb, E. Y., Hanna, E. Y., Hafner, J. H. & Lapotko, D. O. Tunable plasmonic nanobubbles for cell theranostics. Nanotechnology 21, 085102 (2010).
Lukianova-Hleb, E. Y. et al. Improved cellular specificity of plasmonic nanobubbles versus nanoparticles in heterogeneous cell systems. PLoS ONE 7, e34537 (2012).
Lukianova-Hleb, E. Y. et al. Plasmonic nanobubbles rapidly detect and destroy drug-resistant tumors. Theranostics 2, 976–987 (2012).
Pilot Study of AuroLase(TM) Therapy in Refractory and/or Recurrent Tumors of the Head and Neck (Nanospectra Biosciences, 2015); https://clinicaltrials.gov/ct2/show/NCT00848042?term=aurolase%28tm%29&rank=1
Merchant, B. Gold, the noble metal and the paradoxes of its toxicology. Biologicals 26, 49–59 (1998).
Kean, W. F. & Kean, I. R. L. Clinical pharmacology of gold. Inflammopharmacology 16, 112–125 (2008).
Goldstein, N. I., Prewett, M., Zuklys, K., Rockwell, P. & Mendelsohn, J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin. Cancer Res. 1, 1311–1318 (1995).
Wagner, D. S. et al. The in vivo performance of plasmonic nanobubbles as cell theranostic agents in zebrafish hosting prostate cancer xenografts. Biomaterials 31, 7567–7574 (2010).
Chen, H. & Diebold, G. Chemical generation of acoustic waves: a giant photoacoustic effect. Science 250, 963–966 (1995).
Lin, C. P. & Kelly, M. W. Cavitation and acoustic emission around laser-heated microparticles. Appl. Phys. Lett. 72, 2800 (1998).
Lukianova-Hleb, E. Y., Volkov, A. N. & Lapotko, D. O. Laser pulse duration is critical for the generation of plasmonic nanobubbles. Langmuir 30, 7425–7434 (2014).
Reuveni, T., Motiei, M., Romman, Z. & Popovtzer, R. Targeted gold nanoparticles enable molecular CT imaging of cancer: an in vivo study. Int. J. Nanomed. 6, 2859–2864 (2011).
Sano, D. et al. Disruptive TP53 mutation is associated with aggressive disease characteristics in an orthotopic murine model of oral tongue cancer. Clin. Cancer Res. 17, 6658–6670 (2011).
Sharafinski, M. E., Ferris, R. L., Ferrone, S. & Grandis, J. R. Epidermal growth factor receptor targeted therapy of squamous cell carcinoma of the head and neck. Head Neck 32, 1412–1421 (2010).
Maeda, H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 41, 189–207 (2001).
Weissleder, R. A clearer vision for in vivo imaging. Nature Biotechnol. 19, 316–317 (2001).
Acknowledgements
The authors thank E.Y. Hanna and R.J. Karni for the discussion of clinical applications of the technology, A. Hurrell, T. Kelley, E. Batres, D. Wagner, A. Aleknavicius and R. Sulcas for help with experimental equipment, and J. Markovits for assistance with veterinary pathology and surgical training and S. Parminter for copy-editing. E.Y.L.H., Y.S.K., B.E.O. and D.O.L. were supported by grants from the Gillson Longenbaugh Foundation, the National Science Foundation (CBET-1341212) and the National Institutes of Health (R01GM094816).
Author information
Authors and Affiliations
Contributions
E.Y.L.H. conducted PNB experiments, prepared the figures and wrote the manuscript. Y.S.K. conducted the animal experiments and collected animal data. I.B., A.M.G., D.O.L., B.E.O. and E.Y.L.H. discussed the experimental design and results, and clinical applications of the technology. B.E.O. contributed to the conceptual experimental design and organized the animal handling and monitoring. D.O.L. developed the technology and research strategy, designed the experimental setup and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 888 kb)
Supplementary information
Supplementary Movie 1 (WMV 98 kb)
Rights and permissions
About this article
Cite this article
Lukianova-Hleb, E., Kim, YS., Belatsarkouski, I. et al. Intraoperative diagnostics and elimination of residual microtumours with plasmonic nanobubbles. Nature Nanotech 11, 525–532 (2016). https://doi.org/10.1038/nnano.2015.343
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2015.343
This article is cited by
-
Atomically precise photothermal nanomachines
Nature Materials (2024)
-
Advances in photoacoustic imaging aided by nano contrast agents: special focus on role of lymphatic system imaging for cancer theranostics
Journal of Nanobiotechnology (2023)
-
Enrichment and sensing tumor cells by embedded immunomodulatory DNA hydrogel to inhibit postoperative tumor recurrence
Nature Communications (2023)
-
Nanobubble size distribution measurement by interactive force apparatus under an electric field
Scientific Reports (2023)
-
Enhanced postoperative cancer therapy by iron-based hydrogels
Biomaterials Research (2022)