Abstract
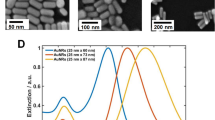
We describe a solution-phase sensor of lipid-protein binding based on localized surface plasmon resonance (LSPR) of silver nanocubes. When silica-coated nanocubes are mixed in a suspension of lipid vesicles, supported membranes spontaneously assemble on their surfaces. Using a standard laboratory spectrophotometer, we calibrated the LSPR peak shift due to protein binding to the membrane surface and then characterized the lipid-binding specificity of a pleckstrin homology domain protein.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Groves, J.T. & Kuriyan, J. Nat. Struct. Mol. Biol. 17, 659–665 (2010).
Baksh, M.M., Kussrow, A.K., Mileni, M., Finn, M.G. & Bornhop, D.J. Nat. Biotechnol. 29, 357–360 (2011).
Baksh, M.M., Jaros, M. & Groves, J.T. Nature 427, 139–141 (2004).
Zheng, G., Patolsky, F., Cui, Y., Wang, W.U. & Lieber, C.M. Nat. Biotechnol. 23, 1294–1301 (2005).
Braun, T. et al. Nat. Nanotechnol. 4, 179–185 (2009).
Cooper, M.A. J. Mol. Recognit. 17, 286–315 (2004).
Beseničar, M., Maček, P., Lakey, J.H. & Anderluh, G. Chem. Phys. Lipids 141, 169–178 (2006).
Dahlin, A. et al. J. Am. Chem. Soc. 127, 5043–5048 (2005).
Galush, W.J. et al. Nano Lett. 9, 2077–2082 (2009).
Jonsson, M.P., Jonsson, P., Dahlin, A.B. & Hook, F. Nano Lett. 7, 3462–3468 (2007).
Baciu, C.L., Becker, J., Janshoff, A. & Sonnichsen, C. Nano Lett. 8, 1724–1728 (2008).
Tao, A., Sinsermsuksakul, P. & Yang, P. Angew. Chem. Int. Ed. 45, 4597–4601 (2006).
Roiter, Y. et al. Langmuir 25, 6287–6299 (2009).
Middleton, E.R. & Rhoades, E. Biophys. J. 99, 2279–2288 (2010).
Garrenton, L.S., Young, S.L. & Thorner, J. Genes Dev. 20, 1946–1958 (2006).
Zhao, C., Du, G.W., Skowronek, K., Frohman, M.A. & Bar-Sagi, D. Nat. Cell Biol. 9, 706–712 (2007).
Fievet, F., Lagier, J.P., Blin, B., Beaudoin, B. & Figlarz, M. Solid State Ionics 32–33 (Part 1), 198–205 (1989).
Sun, Y. & Xia, Y. Science 298, 2176–2179 (2002).
Stöber, W., Fink, A. & Bohn, E. J. Colloid Interface Sci. 26, 62–69 (1968).
Sioss, J.A., Stoermer, R.L., Sha, M.Y. & Keating, C.D. Langmuir 23, 11334–11341 (2007).
Willets, K.A. & Van Duyne, R.P. Annu. Rev. Phys. Chem. 58, 267–297 (2007).
Sherry, L.J. et al. Nano Lett. 5, 2034–2038 (2005).
Mayer, K.M. & Hafner, J.H. Chem. Rev. 111, 3828–3857 (2011).
Forstner, M.B., Yee, C.K., Parikh, A.N. & Groves, J.T. J. Am. Chem. Soc. 128, 15221–15227 (2006).
Bacia, K. & Schwille, P. Nat. Protoc. 2, 2842–2856 (2007).
Chen, Y., Müller, J.D., Eid, J.S. & Gratton, E. in New Trends in Fluorescence Spectroscopy: Applications to Chemical and Life Sciences (eds. Valeur, B. & Brochon, J.-C.), Ch. 14–15, 277–302 (Springer, Berlin, 2001).
Palik, E.D. Handbook of Optical Constants of Solids (Academic, Amsterdam, 1998).
Acknowledgements
This work was supported by the Director, Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under contract no. DE-AC02-05CH11231 (to J.T.G.) and by US National Institutes of Health Research Grant GM21841 (to J.T.). P.Y. would like to acknowledge the support from King Abdulaziz University.
Author information
Authors and Affiliations
Contributions
H.-J.W. and J.T.G. conceived the solution-phase nanocube sensor strategy. H.-J.W. implemented the experiments, J.H. synthesized nanocubes and performed TEM, W.-C.L. performed FCS measurements, C.R. performed LSPR simulations and Z.L. and E.S. prepared Ste5 proteins. H.-J.W., C.R. and J.T.G. wrote the manuscript. J.T.G., J.T. and P.Y. supervised the project. All authors discussed the results and commented on the manuscript at all stages.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Table 1 and Supplementary Discussion (PDF 1434 kb)
Rights and permissions
About this article
Cite this article
Wu, HJ., Henzie, J., Lin, WC. et al. Membrane-protein binding measured with solution-phase plasmonic nanocube sensors. Nat Methods 9, 1189–1191 (2012). https://doi.org/10.1038/nmeth.2211
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.2211
This article is cited by
-
Surface Plasmon Excitation: Theory, Configurations, and Applications
Plasmonics (2023)
-
Geometric pinning and antimixing in scaffolded lipid vesicles
Nature Communications (2020)
-
Self-assembly of robust gold nanoparticle monolayer architectures for quantitative protein interaction analysis by LSPR spectroscopy
Analytical and Bioanalytical Chemistry (2020)
-
Metal-organic frameworks for CO2 photoreduction
Frontiers in Energy (2019)
-
Hetero-Multivalency of Pseudomonas aeruginosa Lectin LecA Binding to Model Membranes
Scientific Reports (2018)