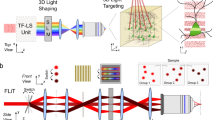
Abstract
Light-gated ion channels and pumps have made it possible to probe intact neural circuits by manipulating the activity of groups of genetically similar neurons. What is needed now is a method for precisely aiming the stimulating light at single neuronal processes, neurons or groups of neurons. We developed a method that combines generalized phase contrast with temporal focusing (TF-GPC) to shape two-photon excitation for this purpose. The illumination patterns are generated automatically from fluorescence images of neurons and shaped to cover the cell body or dendrites, or distributed groups of cells. The TF-GPC two-photon excitation patterns generated large photocurrents in Channelrhodopsin-2–expressing cultured cells and neurons and in mouse acute cortical slices. The amplitudes of the photocurrents can be precisely modulated by controlling the size and shape of the excitation volume and, thereby, be used to trigger single action potentials or trains of action potentials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Penfield, W. & Rasmussen, T. The cerebral cortex of man: a clinical study of localization of function. J. Am. Med. Assoc. 144, 1412–1700 (1950).
Nagel, G. et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 100, 13940–13945 (2003).
Gunaydin, L.A. et al. Ultrafast optogenetic control. Nat. Neurosci 13, 387–392 (2010).
Boyden, E.S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Wang, H. et al. High-speed mapping of synaptic connectivity using photostimulation in Channelrhodopsin-2 transgenic mice. Proc. Natl. Acad. Sci. USA 104, 8143–8148 (2007).
Grossman, N. et al. Multi-site optical excitation using ChR2 and micro-LED array. J. Neural Eng. 7, 16004 (2010).
Aravanis, A.M. et al. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural Eng. 4, S143–S156 (2007).
Gradinaru, V. et al. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J. Neurosci. 27, 14231–14238 (2007).
Gradinaru, V., Mogri, M., Thompson, K.R., Henderson, J.M. & Deisseroth, K. Optical deconstruction of parkinsonian neural circuitry. Science 324, 354–359 (2009).
Huber, D. et al. Sparse optical microstimulation in barrel cortex drives learned behaviour in freely moving mice. Nature 451, 61–64 (2008).
Lagali, P.S. et al. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat. Neurosci. 11, 667–675 (2008).
Feldbauer, K. et al. Channelrhodopsin-2 is a leaky proton pump. Proc. Natl. Acad. Sci. USA 106, 12317–12322 (2009).
Rickgauer, J.P. & Tank, D.W. Two-photon excitation of channelrhodopsin-2 at saturation. Proc. Natl. Acad. Sci. USA 106, 15025–15030 (2009).
Andrasfalvy, B.K., Zemelman, B.V., Tang, J. & Vaziri, A. Two-photon single-cell optogenetic control of neuronal activity by sculpted light. Proc. Natl. Acad. Sci. USA 107, 11981–11986 (2010).
Curtis, J.E., Koss, B.A. & Grier, D.G. Dynamic holographic optical tweezers. Opt. Commun. 207, 169 (2002).
Lutz, C. et al. Holographic photolysis of caged neurotransmitters. Nat. Methods 5, 821–827 (2008).
Zahid, M. et al. Holographic photolysis for multiple cell stimulation in mouse hippocampal slices. PLoS ONE 5, e9431 (2010).
Oron, D., Tal, E. & Silberberg, Y. Scanningless depth-resolved microscopy. Opt. Express 13, 1468–1476 (2005).
Papagiakoumou, E., de Sars, V., Oron, D. & Emiliani, V. Patterned two-photon illumination by spatiotemporal shaping of ultrashort pulses. Opt. Express 16, 22039–22047 (2008).
Papagiakoumou, E., de Sars, V., Emiliani, V. & Oron, D. Temporal focusing with spatially modulated excitation. Opt. Express 17, 5391–5401 (2009).
Golan, L., Reutsky, I., Farah, N. & Shoham, S. Design and characteristics of holographic neural photo-stimulation systems. J. Neural Eng. 6, 66004 (2009).
Glückstad, J. Phase contrast image synthesis. Opt. Commun. 130, 225 (1996).
Rodrigo, P.J., Daria, V.R. & Glückstad, J. Real-time three-dimensional optical micromanipulation of multiple particles and living cells. Opt. Lett. 29, 2270–2272 (2004).
Rodrigo, P.J., Palima, D. & Glückstad, J. Accurate quantitative phase imaging using generalized phase contrast. Opt. Express 16, 2740–2751 (2008).
Hopt, A. & Neher, E. Highly nonlinear photodamage in two-photon fluorescence microscopy. Biophys. J. 80, 2029–2036 (2001).
Wang, S. et al. All optical interface for parallel, remote, and spatiotemporal control of neuronal activity. Nano Lett. 7, 3859–3863 (2007).
Guo, Z.V., Hart, A.C. & Ramanathan, S. Optical interrogation of neural circuits in Caenorhabditis elegans. Nat. Methods 6, 891–896 (2009).
Shoham, S., O'Connor, D.H., Sarkisov, D.V. & Wang, S.S. Rapid neurotransmitter uncaging in spatially defined patterns. Nat. Methods 2, 837–843 (2005).
Losavio, B.E., Iyer, V., Patel, S. & Saggau, P. Acousto-optic laser scanning for multi-site photo-stimulation of single neurons in vitro. J. Neural Eng. 7, 045002 (2010).
Kirkby, P.A., Srinivas Nadella, K.M. & Silver, R.A. A compact Acousto-Optic Lens for 2D and 3D femtosecond based 2-photon microscopy. Opt. Express 18, 13721–13745 (2010).
Glückstad, J. & Mogensen, P.C. Optimal phase contrast in common-path interferometry. Appl. Opt. 40, 268–282 (2001).
Palima, D. & Glückstad, J. Multi-wavelength spatial light shaping using generalized phase contrast. Opt. Express 16, 1331–1342 (2008).
Nagel, G. et al. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol. 15, 2279–2284 (2005).
Arenkiel, B.R. et al. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron 54, 205–218 (2007).
Otsu, Y. et al. Optical monitoring of neuronal activity at high frame rate with a digital random-access multiphoton (RAMP) microscope. J. Neurosci. Methods 173, 259–270 (2008).
Acknowledgements
We thank I. Perch-Nielsen for the phase-contrast filter layout design, E. Schwartz for genotyping ChR2-YFP mice, S. Wiese, Z. Fu and M. Viesel for generating cDNA constructs, A. Triller, T. Gally, K. Spence, A. Burgo and K. Zylbersztejn for cell culture preparation, all members of the Neurophysiology and New Microscopy Laboratory for comments and technical help, D. Oron, D. Palima, S. Dieudonné, M. Diana and G. Fortin for helpful discussions, J. Feldmann for critical reading of the paper, Spectra-Physics, Inc. for loan of the high-power laser, and Phasics S.A. for providing the phase-analyzer software. V.E. was supported by the European Science Foundation and the Centre National de la Recherche Scientifique through the European Young Investigator program and by the European Network of Neuroscience Institutes (LSHM-CT-2005-19063). E.P. and V.E. were supported by the European Commission FP6 Specific Targeted Project Photolysis (LSHM-CT-2007-037765). E.P. was supported by the Fondation pour la Recherche Médicale. F.A. was supported by the European doctoral school Frontières du Vivant. A.B. was supported by Paris School of Neuroscience. J.G. was supported by the Danish Technical Scientific Research Councils (09-060742), E.Y.I. was supported by the US National Institutes of Health Nanomedicine Development Center for the Optical Control of Biological Function (PN2EY018241) and the Paris School of Neuroscience. E.Y.I. and V.E. were supported by Human Frontier Science Program (RGP0013/2010).
Author information
Authors and Affiliations
Contributions
E.P. set up and characterized the optical properties of the TF-GPC microscope; F.A. and A.B. implemented the optical microscope with electrophysiological recording; E.P., F.A. and A.B. performed the experiments on cell cultures and brain slices; F.A. and A.B. analyzed the experiments on cell cultures and brain slices; V.d.S. developed the software; J.G. contributed to the set up of the GPC microscope; E.Y.I. contributed in conceiving the experiments in cultured cells and brain slices and discussed the results; E.Y.I. and V.E. prepared the manuscript; and V.E. conceived and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Supplementary Note 1 (PDF 1042 kb)
Rights and permissions
About this article
Cite this article
Papagiakoumou, E., Anselmi, F., Bègue, A. et al. Scanless two-photon excitation of channelrhodopsin-2. Nat Methods 7, 848–854 (2010). https://doi.org/10.1038/nmeth.1505
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1505
This article is cited by
-
A micro-LED array based platform for spatio-temporal optogenetic control of various cardiac models
Scientific Reports (2023)
-
Optogenetics for light control of biological systems
Nature Reviews Methods Primers (2022)
-
All-optical interrogation of neural circuits in behaving mice
Nature Protocols (2022)
-
Real-time precision opto-control of chemical processes in live cells
Nature Communications (2022)
-
Kalium channelrhodopsins are natural light-gated potassium channels that mediate optogenetic inhibition
Nature Neuroscience (2022)