Abstract
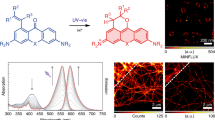
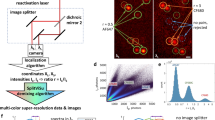
The reliance of modern microscopy techniques on photoactivatable fluorescent proteins prompted development of mCherry variants that are initially dark but become red fluorescent after violet-light irradiation. Using ensemble and single-molecule characteristics as selection criteria, we developed PAmCherry1 with excitation/emission maxima at 564/595 nm. Compared to other monomeric red photoactivatable proteins, it has faster maturation, better pH stability, faster photoactivation, higher photoactivation contrast and better photostability. Lack of green fluorescence and single-molecule behavior make monomeric PAmCherry1 a preferred tag for two-color diffraction-limited photoactivation imaging and for super-resolution techniques such as one- and two-color photoactivated localization microscopy (PALM). We performed PALM imaging using PAmCherry1-tagged transferrin receptor expressed alone or with photoactivatable GFP–tagged clathrin light chain. Pair correlation and cluster analyses of the resulting PALM images identified ≤200 nm clusters of transferrin receptor and clathrin light chain at ≤25 nm resolution and confirmed the utility of PAmCherry1 as an intracellular probe.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
26 February 2009
NOTE: In the version of this article initially published, the affiliations listed for Fedor V. Subach were incorrect. The error has been corrected in the HTML and PDF versions of the article.
References
Lukyanov, K.A., Chudakov, D.M., Lukyanov, S. & Verkhusha, V.V. Innovation: Photoactivatable fluorescent proteins. Nat. Rev. Mol. Cell Biol. 6, 885–891 (2005).
Hell, S.W. Far-field optical nanoscopy. Science 316, 1153–1158 (2007).
Betzig, E. et al. Imaging intracellular fluorescent proteins at nanometer resolution. Science 313, 1642–1645 (2006).
Hess, S.T., Girirajan, T.P. & Mason, M.D. Ultra-high resolution imaging by fluorescence photoactivation localization microscopy. Biophys. J. 91, 4258–4272 (2006).
Rust, M.J., Bates, M. & Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 3, 793–795 (2006).
Egner, A. et al. Fluorescence nanoscopy in whole cells by asynchronous localization of photoswitching emitters. Biophys. J. 93, 3285–3290 (2007).
Flors, C. et al. A stroboscopic approach for fast photoactivation-localization microscopy with Dronpa mutants. J. Am. Chem. Soc. 129, 13970–13977 (2007).
Verkhusha, V.V. & Sorkin, A. Conversion of the monomeric red fluorescent protein into a photoactivatable probe. Chem. Biol. 12, 279–285 (2005).
Ando, R., Hama, H., Yamamoto-Hino, M., Mizuno, H. & Miyawaki, A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc. Natl. Acad. Sci. USA 99, 12651–12656 (2002).
Chudakov, D.M. et al. Kindling fluorescent proteins for precise in vivo photolabeling. Nat. Biotechnol. 21, 191–194 (2003).
Wiedenmann, J. et al. EosFP, a fluorescent marker protein with UV-inducible green-to-red fluorescence conversion. Proc. Natl. Acad. Sci. USA 101, 15905–15910 (2004).
Gurskaya, N.G. et al. Engineering of a monomeric green-to-red photoactivatable fluorescent protein induced by blue light. Nat. Biotechnol. 24, 461–465 (2006).
Patterson, G.H. & Lippincott-Schwartz, J. A photoactivatable GFP for selective photolabeling of proteins and cells. Science 297, 1873–1877 (2002).
Chudakov, D.M. et al. Photoswitchable cyan fluorescent protein for protein tracking. Nat. Biotechnol. 22, 1435–1439 (2004).
Ando, R., Mizuno, H. & Miyawaki, A. Regulated fast nucleocytoplasmic shuttling observed by reversible protein highlighting. Science 306, 1370–1373 (2004).
Stiel, A.C. et al. Generation of monomeric reversibly switchable red fluorescent proteins for far-field fluorescence nanoscopy. Biophys. J. 95, 2989–2997 (2008).
Shaner, N.C. et al. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat. Biotechnol. 22, 1567–1572 (2004).
Shu, X., Shaner, N.C., Yarbrough, C.A., Tsien, R.Y. & Remington, S.J. Novel chromophores and buried charges control color in mFruits. Biochemistry 45, 9639–9647 (2006).
Verkhusha, V.V. & Lukyanov, K.A. The molecular properties and applications of Anthozoa fluorescent proteins and chromoproteins. Nat. Biotechnol. 22, 289–296 (2004).
Huebers, H.A., Huebers, E., Josephson, B. & Csiba, E. A highly efficient chemical isolation procedure for the rat placental transferrin receptor. Biochim. Biophys. Acta 991, 30–35 (1989).
Gallione, C.J. & Rose, J.K. A single amino acid substitution in a hydrophobic domain causes temperature sensitive cell-surface transport of a mutant viral glycoprotein. J. Virol. 54, 374–382 (1985).
McGraw, T.E. & Maxfield, F.R. Human transferrin receptor internalization is partially dependent upon an aromatic amino acid on the cytoplasmic domain. Cell Regul. 1, 369–377 (1990).
Hopkins, C.R. The appearance and internalization of transferrin receptors at the margins of spreading human tumor cells. Cell 40, 199–208 (1985).
Shroff, H. et al. Dual-color super-resolution imaging of genetically expressed probes within individual adhesion complexes. Proc. Natl. Acad. Sci. USA 104, 20308–20313 (2007).
Manley, S. et al. High-density mapping of single-molecule trajectories with photoactivated localization microscopy. Nat. Methods 5, 155–157 (2008).
Pearse, B.M.F. & Robinson, M.S. Clathrin, adaptors, and sorting. Annu. Rev. Cell Biol. 6, 151–171 (1990).
Heuser, J.E. & Anderson, R.G. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 108, 389–400 (1989).
Kirchhausen, T. Clathrin. Annu. Rev. Biochem. 69, 699–727 (2000).
Habuchi, S. et al. Reversible single-molecule photoswitching in the GFP-like fluorescent protein Dronpa. Proc. Natl. Acad. Sci. USA 102, 9511–9516 (2005).
Bock, H. et al. Two-color far-field fluorescence nanoscopy based on photoswitchable emitters. Appl. Phys. B 88, 161–165 (2007).
Acknowledgements
We thank R. Tsien (University of California at San Diego) for providing the pRSETB-mCherry plasmid, J. Wiedenmann (University of Ulm) for providing plasmids encoding EosFP variants, J. Zhang for assistance with flow cytometry, O. Subach for assistance with cell culture and imaging, and E. Betzig and H. Hess for assistance with PALM experiments, analysis and discussion. This work was supported by grants GM070358 and GM073913 from the US National Institutes of Health to V.V.V.
Author information
Authors and Affiliations
Contributions
F.V.S. developed proteins and characterized them in vitro. G.H.P., S.M. and J.M.G. characterized proteins in mammalian cells. J.L.-S. and V.V.V. designed and planned the project. G.H.P. and V.V.V. wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Table 1, Supplementary Methods (PDF 9095 kb)
Rights and permissions
About this article
Cite this article
Subach, F., Patterson, G., Manley, S. et al. Photoactivatable mCherry for high-resolution two-color fluorescence microscopy. Nat Methods 6, 153–159 (2009). https://doi.org/10.1038/nmeth.1298
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1298
This article is cited by
-
Bright and stable monomeric green fluorescent protein derived from StayGold
Nature Methods (2024)
-
Super-resolved trajectory-derived nanoclustering analysis using spatiotemporal indexing
Nature Communications (2023)
-
Growth-rate dependency of ribosome abundance and translation elongation rate in Corynebacterium glutamicum differs from that in Escherichia coli
Nature Communications (2023)
-
Targeting mitochondrial shape: at the heart of cardioprotection
Basic Research in Cardiology (2023)
-
The emergence of molecular systems neuroscience
Molecular Brain (2022)