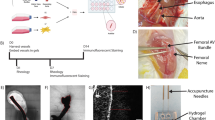
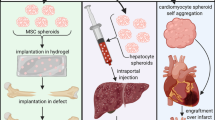
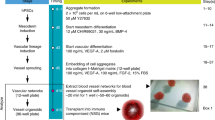
Abstract
The complexity of the angiogenic cascade limits cellular approaches to studying angiogenic endothelial cells (ECs). In turn, in vivo assays do not allow the analysis of the distinct cellular behavior of ECs during angiogenesis. Here we show that ECs can be grafted as spheroids into a matrix to give rise to a complex three-dimensional network of human neovessels in mice. The grafted vasculature matures and is connected to the mouse circulation. The assay is highly versatile and facilitates numerous applications including studies of the effects of different cytokines on angiogenesis. Modifications make it possible to study human lymphangiogenic processes in vivo. EC spheroids can also be coimplanted with other cell types for tissue engineering purposes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
National Cancer Institute (USA). Angiogenesis initiative fueling collaboration. Cancer Bull. 3 (9), 1–6 (2006).
Ausprunk, D.H., Knighton, D.R. & Folkman, J. Vascularization of normal and neoplastic tissues grafted to the chick chorioallantois. Role of host and preexisting graft blood vessels. Am. J. Pathol. 79, 597–628 (1975).
Korff, T., Krauss, T. & Augustin, H.G. Three-dimensional spheroidal culture of cytotrophoblast cells mimics the phenotype and differentiation of cytotrophoblasts from normal and preeclamptic pregnancies. Exp. Cell Res. 297, 415–423 (2004).
Jain, R.K., Schlenger, K., Hockel, M. & Yuan, F. Quantitative angiogenesis assays: progress and problems. Nat. Med. 3, 1203–1208 (1997).
Kenyon, B.M. et al. A model of angiogenesis in the mouse cornea. Invest. Ophthalmol. Vis. Sci. 37, 1625–1632 (1996).
Norrby, K. In vivo models of angiogenesis. J. Cell. Mol. Med. 10, 588–612 (2006).
Passaniti, A. et al. A simple, quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin, and fibroblast growth factor. Lab. Invest. 67, 519–528 (1992).
Korff, T. & Augustin, H.G. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J. Cell Biol. 143, 1341–1352 (1998).
Ferrara, N. VEGF and the quest for tumour angiogenesis factors. Nat. Rev. Cancer 2, 795–803 (2002).
Presta, M. et al. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 16, 159–178 (2005).
Grunewald, M. et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell 124, 175–189 (2006).
Drevs, J. et al. Effects of PTK787/ZK 222584, a specific inhibitor of vascular endothelial growth factor receptor tyrosine kinases, on primary tumor, metastasis, vessel density, and blood flow in a murine renal cell carcinoma model. Cancer Res. 60, 4819–4824 (2000).
Kriehuber, E. et al. Isolation and characterization of dermal lymphatic and blood endothelial cells reveal stable and functionally specialized cell lineages. J. Exp. Med. 194, 797–808 (2001).
Rouwkema, J., de Boer, J. & van Blitterswijk, C.A. Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng. 12, 2685–2693 (2006).
Enis, D.R. et al. Induction, differentiation, and remodeling of blood vessels after transplantation of Bcl-2-transduced endothelial cells. Proc. Natl. Acad. Sci. USA 102, 425–430 (2005).
Yang, J. et al. Telomerized human microvasculature is functional in vivo. Nat. Biotechnol. 19, 219–224 (2001).
Choong, C.S., Hutmacher, D.W. & Triffitt, J.T. Co-culture of bone marrow fibroblasts and endothelial cells on modified polycaprolactone substrates for enhanced potentials in bone tissue engineering. Tissue Eng. 12, 2521–2531 (2006).
Koike, N. et al. Tissue engineering: creation of long-lasting blood vessels. Nature 428, 138–139 (2004).
Levenberg, S. et al. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 23, 879–884 (2005).
Fischbach, C. et al. Engineering tumors with 3D scaffolds. Nat. Methods 4, 855–860 (2007).
Ford, M.C. et al. A macroporous hydrogel for the coculture of neural progenitor and endothelial cells to form functional vascular networks in vivo. Proc. Natl. Acad. Sci. USA 103, 2512–2517 (2006).
Nor, J.E. et al. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab. Invest. 81, 453–463 (2001).
Skovseth, D.K., Yamanaka, T., Brandtzaeg, P., Butcher, E.C. & Haraldsen, G. Vascular morphogenesis and differentiation after adoptive transfer of human endothelial cells to immunodeficient mice. Am. J. Pathol. 160, 1629–1637 (2002).
Skovseth, D.K., Kuchler, A.M. & Haraldsen, G. The HUVEC/Matrigel assay: an in vivo assay of human angiogenesis suitable for drug validation. Methods Mol. Biol. 360, 253–268 (2007).
Levenberg, S., Golub, J.S., Amit, M., Itskovitz-Eldor, J. & Langer, R. Endothelial cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 99, 4391–4396 (2002).
Wang, Z.Z. et al. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat. Biotechnol. 25, 317–318 (2007).
Au, P. et al. Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood 111, 1302–1305 (2008).
Wong, C., Inman, E., Spaethe, R. & Helgerson, S. Fibrin-based biomaterials to deliver human growth factors. Thromb. Haemost. 89, 573–582 (2003).
Alitalo, K., Tammela, T. & Petrova, T.V. Lymphangiogenesis in development and human disease. Nature 438, 946–953 (2005).
Eiselt, P. et al. Development of technologies aiding large-tissue engineering. Biotechnol. Prog. 14, 134–140 (1998).
Acknowledgements
This work was supported by grants from the German Research Council (Deutsche Forschungsgemeinschaft, AU83/10-1), the European Union (LSHG-CT-2004-503573), the Austrian Science Fund (FWF S9404-B11), the State of Baden-Württemberg Kompetenznetzwerk Biomaterialien and the Fördergesellschaft der Klinik für Tumorbiologie Freiburg.
Author information
Authors and Affiliations
Contributions
A.A., A.M.L., H.W., S.C., M.H., G.B.S. and H.G.A. conceived and designed the experiments. A.A., A.M.L., H.W., A.M.B., A.B., K.I., T.K., H.Z., C.O., R.G., G.F. and M.H. performed experiments. A.A., A.M.L., H.W., M.H., S.C. and H.G.A. contributed to data analysis. A.A., A.M.L., H.W., M.H. and H.G.A. contributed to the writing of the manuscript. All authors discussed and commented on the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Methods (PDF 3008 kb)
Rights and permissions
About this article
Cite this article
Alajati, A., Laib, A., Weber, H. et al. Spheroid-based engineering of a human vasculature in mice. Nat Methods 5, 439–445 (2008). https://doi.org/10.1038/nmeth.1198
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth.1198
This article is cited by
-
Hypoxia-induced P4HA1 overexpression promotes post-ischemic angiogenesis by enhancing endothelial glycolysis through downregulating FBP1
Journal of Translational Medicine (2024)
-
Methods for vascularization and perfusion of tissue organoids
Mammalian Genome (2022)
-
An in vivo model allowing continuous observation of human vascular formation in the same animal over time
Scientific Reports (2021)
-
Surface acoustic wave (SAW) techniques in tissue engineering
Cell and Tissue Research (2021)
-
Prevascularized Micro-/Nano-Sized Spheroid/Bead Aggregates for Vascular Tissue Engineering
Nano-Micro Letters (2021)